Blood is a depreciating commodity. Current UK guidelines assign donated blood a shelf life of 35 days at 2-6°C, although some studies suggest that even this is too long for red blood cells.1 Stocks of blood in the UK for transfusion rarely exceed enough for one week. If blood could safely be preserved for longer, this could be significantly increased.
Freeze drying offers the opportunity of stabilising blood for storage. In times of excess supply, blood could be stored instead of discarded. Stockpiles could be made of rare blood types. Packets of dried blood would be easily transportable to trauma sites and battlefields where they could be rehydrated and administered quickly to save lives. Long term storage of blood also offers the possibility of auto transfusion, whereby patients can store their own blood for future transfusions, hugely decreasing the risk of infection and other complications.
The factors of blood
After donation, blood is separated into factors for storage, each of which has a different shelf life and different storage requirements. Plasma, used to maintain blood pressure during operations or after severe loss of blood, can be safely frozen or freeze dried. Fresh Frozen Plasma (FFP) is routinely used in hospitals, but can be expensive or impossible to transport to the scene of accident and injury because it needs to be kept in a freezer.
The freeze drying of plasma has made it much easier to supply plasma in trauma and warfare situations. The French Military Blood Bank has been producing and using freeze dried plasma (FDP) in overseas operations since 1994. Not only is it possible to store and transport it at room temperature, it is also easier to use than FFP since rehydrating the dried product takes less time than thawing the frozen product. Clinically, the efficacy of FDP is equivalent to FFP.2
Platelets, with a refrigerated shelf life of only five days, can be freeze dried for in vitro use, but research is on-going for in vivo applications.3 White blood cells are not used for transfusion, but they can be safely frozen. However, there have been no reports of 100% success for either freezing or freeze drying red blood cells.
Biological material
Freeze drying is a mature technology. It was first used commercially in the 1950s and today is routinely used in the food industry to preserve coffee, instant soups and soft fruit such as raspberries. Freeze drying, also known as lyophilisation, is also widely used in the pharmaceutical industry to preserve vaccines and to dry therapeutic drugs so that they can be formed into capsules.
Current research focuses on freeze drying biological material such as bacteria, proteins, collagen, adult stem cells and red blood cells, often for applications in regenerative medicine.4 The challenge is to freeze dry biomaterials so that they reconstitute reliably, preserving both form and function. For example, if a protein unfolds during freeze drying it is very unlikely to regain its folded conformation when reconstituted. Usefully, it has been found that if certain sugars are added to the protein before it is dried, they can prevent the molecule from unfolding during drying.5 Freeze drying, or lyophilisation, is the process of freezing a material then sublimating the frozen liquid, followed by the removal of unfrozen water, resulting in a stable, dried product that can be stored at room temperature. It proceeds in four main stages: protection, freezing, primary drying and secondary drying.
For the successful lyophilisation of biological products, the focus is on the first and second stages – adding appropriate buffers that protect the material during freezing, then ensuring the freezing proceeds at the correct rate so that ice crystal formation causes minimal damage to the dried material. If the material is frozen quickly, small ice crystals will form; if the material is frozen slowly, larger and potentially more damaging crystals will form. The optimum freezing rate varies for each material. As the ice crystals form, the solutes in the cell collect and concentrate, which can cause damage to cells. This can be minimised by the introduction of a cryoprotectant, such as a sugar, amino acid or glycerol. However, damage can also occur during the drying cycles.
The primary drying stage involves the removal of the solvent from the frozen product. During primary drying, the product is placed in a pressurised drying chamber. The shelf temperature and vacuum are controlled to achieve sublimation, whereby the frozen solvent sublimes straight into a vapour without passing through the liquid stage. At the same time, the product temperature is kept below the critical temperature. The critical temperature is different for every product, but could typically be in the range of -10 to -50°C.
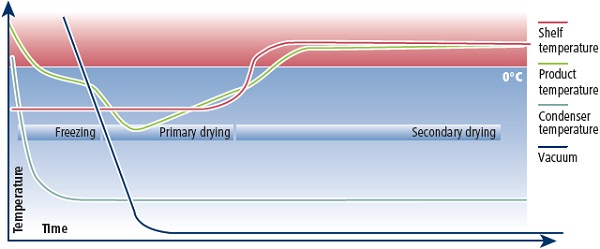
Figure 1: Variable studied for the freeze drying of red blood cells.
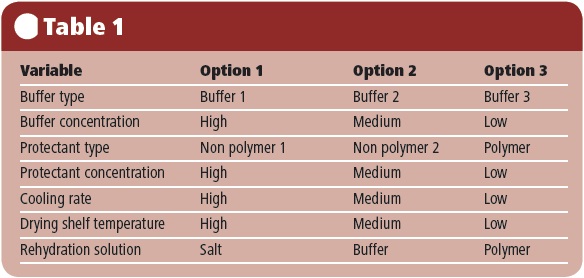
Table 1: The Freeze Drying Cycle, showing variations in temperature and pressure during the freezing and drying stages.
After primary drying, the product is at less risk of melting and the temperature can be increased. During secondary drying, the chamber pressure is reduced to a minimum to desorb any remaining unfrozen water.
So why do red blood cells (RBCs) present such a challenge? RBCs, or erythrocytes, are biconcave discs 7-8μm in diameter. The function of these non-nucleated cells is to carry oxygenated haemoglobin around the body. Their large surface area relative to volume facilitates the diffusion of oxygen in and out of the cell. RBCs are very flexible and can flow through capillaries half their own diameter.
The cytoskeleton of mammalian RBCs is composed of two layers: one made primarily of proteins and the other of lipids, namely cholesterol and phospholipids. It is the proteins that are responsible for the flexibility of the cells. Damage to the structure of RBCs can be caused at every stage of the freeze drying process.
During the freezing stage, damage can be caused from ice crystal growth, from pH gradients and from freeze-concentration and osmotic effects. Drying damage is typically caused by the physical action of the ice removal on the cell membrane and by dehydration causing deformation of the cells.
Perhaps surprisingly, damage can also be caused to the cells on rehydration. As water is added, the concentration and pH levels often vary throughout the material, causing localised hypotonic, acidic or alkaline regions, leading to rupture or lysis of the RBCs. Finally, there may be other changes induced by freezing, drying and rehydration that are more subtle than such obvious physical or chemical changes, but yet may also have an impact on the potential use of the RBCs in a clinical setting.
Arguably, the two most basic hurdles for the formulation and freeze-drying process to overcome are the RBC survival rate and the level of haemoglobin oxidation. Physical damage to RBCs is relatively simple to measure in terms of haemoglobin leakage, while the oxidation state of the haemoglobin itself may be quantified using a straightforward assay.
A recent UK study by Biopharma Technology (BTL) and the University of Cambridge focused on improving the freeze drying of red blood cells. The study was funded by the UK’s Technology Strategy Board (TSB) and investigated the effect of a series of formulation and process variables on the survival of RBC after freeze-drying and rehydration.
The level of haemoglobin release was quantified in terms of release in the separated supernatant, compared with that in the pellet. Haemoglobin oxidation was measured by UV-visible spectrophotometry, comparing Hb with met-Hb and oxy-Hb. Variables included using different types and concentrations of buffer and protectant, different cooling rates, a range of shelf temperatures during primary drying, and different solutions for rehydration (see Table 1).
Novel biopolymer to aid protectant absorption
Trehalose is a sugar that has previously showed promise in protecting RBCs through cryopreservation and lyophilisation.6 However, for the protectant to be effective it has not only to be mixed with the cells, but must actually permeate them.
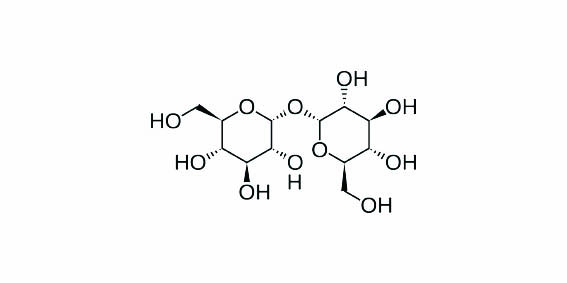
Figure 2: Structure of trehalose.
Trehalose is a non-reducing disaccharide of glucose, and disaccharides are normally impermeable to the phospholipid bilayer of RBC membranes. Scientists at the University of Cambridge used novel synthetic biopolymers to increase the RBC membrane permeability to the trehalose.
Previous work has been done on naturally-derived and synthetic peptides to improve RBC membrane permeability for drug delivery, but there have been problems with safety and production using these peptides. The amphipathic polymers synthesised by the Cambridge group have weakly ionisable carbon groups and hydrophobic side chains. They make an interesting alternative to synthetic peptides because of their ability to mimic the viral peptide structure and their ability to permeate the RBC membranes.
The Cambridge scientists tested different forms of the biopolymer, grafting them with hydrophobic amino groups, as well as testing the unmodified biopolymer backbone. They found that the cellular uptake of trehalose differed depending on which form of the biopolymer was used, and also varied with the polymer concentration, pH, the external trehalose concentration, incubation temperature and time.7 Optimisation of these parameters yielded an improvement of RBC survival under freezing of up to 20%. Additionally, the biopolymer mediated membrane permeability was shown to be rapidly and completely reversible via washing with phosphate buffered saline.8
The results of the study showed that levels of red blood cell survival of 96% through freeze drying and rehydration were achievable for the combination of variables tested. Surprisingly, this was achieved in the absence of the biopolymer, which implies that the intracellular loading of the trehalose may not be necessary. However the oxidation level of the haemoglobin was rather high, at 60%.
The addition of the biopolymer led to a reduction in haemoglobin oxidation below detectible levels, typically below 10%. However, the maximum RBC survival rate with the biopolymer was 85%. The prevention of the Hb oxidation may have been due to the direct action of the polymer itself, or to the intracellular presence of the trehalose facilitated by the polymer. Valuable lessons were learned from this study that will be applied to further studies.
Next steps
Being able to store blood while it is fresh and keeping it in a stable, readily transportable state at room temperature promises immense clinical advantages. The freeze drying of red blood cells would give health organisations the opportunity to stockpile rare blood types. It would make autologous transfusion far more accessible, whereby patients could donate and store their own blood before operations, removing the risk of infection, and enabling them to donate blood far enough in advance of surgery for them to recover fully from the donation. Finally, the ability to transport freeze dried red blood cells to emergency sites could save lives in military and disaster situations, and make it much easier to transport and store blood in parts of the world where power supplies are not reliable.
This study and follow up work from it are bringing us closer to the successful freeze drying of red blood cells.
References
1. E. A. Hod and S. L. Spitalnik, Transfusion, 2011, 51(4), 881.
2. C. Martinaud et al, J. Trauma, 2011, 71(6), 1761.
3. E. B. Davidow et al, J. Veter. Emerg. and Critical Care, 2012, 22(1), 116.
4. I. Cook, Pharmaceutical Technology Europe, 2011, 23(7).
5. K. R. Ward et al, International Journal of Pharmaceutics, 1999, 187, 153.
6. Y. Zhuang and J. H. Liu, Zhongguo Shi Yan Xue Ye Xue Za Zhi, 2006, 14(5), 1061.
7. A. L. Lynch et al, Biomaterials, 2010, 31(23), 6096.
8. Current level of blood stocks in the UK: http://www.blood.co.uk/StockGraph/stocklevelstandard.aspx
Katriona Scoffin is a freelance writer working for Biopharma Technology, based in Cambridge, UK.




