Batch reactors have been heavily used for fine chemical manufacture for centuries. However, while many processes work extremely well in batch mode, certain types of reactions are less successful. A few of these are difficult or dangerous to scale up. In those cases, fine chemical manufacturers have increasingly turned towards the use of continuous flow reactors.
A continuous flow reactor is in the order of 10,000 times smaller than a batch reactor with the same throughput performance. If the reaction and chemistry work for both types of reactors, a 10,000L batch reactor can be replaced by a 1L flow reactor. The resulting high surface-to-volume ratio is an important feature of flow reactors, along with their high heat and mass transfer capabilities.
Another feature of flow reactors is the narrow and constant residence time, which is especially beneficial for products that decompose when exposed for too long to the reaction conditions required to run the wanted reaction. The continuous nature of the processing further adds to the advantage because increasing batch size simply involves running a flow reactor for longer. It also reduces efforts in the long run as sequential scale-ups to increasingly large batch reactors are not needed.
Continuous flow reactors have been shown to offer a range of advantages when applied in the right situations. These include better reproducibility, reduced development, improved safety, and even access to molecules that cannot otherwise be made on a large scale.
Improved reproducibility
One of the key advantages of a continuous flow process is that it often gives better reproducibility from run to run than from batch to batch in a traditional reactor; it is much easier to achieve a defined and homogenous temperature level with a flow reactor. This is particularly important for highly exothermic reactions. The heat of reaction is easier to dissipate with a small flow reactor than with a large batch reactor. A common problem of highly exothermic reactions run in batch reactors is non-uniform temperature distribution, so-called ‘hotspots’. This frequently leads to problems like reduced yields, more impurities during scale-up or variability in yield from batch to batch.
Take the production of tetracyanoethylene oxide from tetracyanoethylene as an example. When using hydrogen peroxide as the oxidant, the exotherm is difficult to control at a 25g scale, never mind in larger batches. At that 25g scale, the typical yield is 65%. When the scale is increased to 200g, this drops to a variable 0 to 23%. The optimum reaction time is 10 minutes and, in addition to the hazards of the exotherm, the product decomposes on contact with water, generating hydrogen cyanide, (Figure 1).
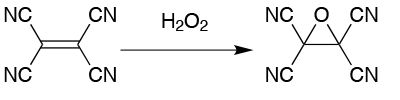
This product was an obvious candidate for transferring to flow and the process development proved surprisingly straightforward. No changes were needed to solvent or concentrations and no optimisation was needed. A continuous workup system gave a two-stage continuous process, an immediate quench with water providing a constant reaction time. In the flow process, the optimum reaction time is three minutes, independent of scale, with a productivity of 100g/h. Without optimisation, a yield of 47% of colourless product is achieved, with similar results in several production runs.
Less development effort
Another drawback to batch processing is the amount of development time that can be required to transfer a reaction from the lab scale to a large reactor. While sometimes lab conditions are appropriate and successful at larger scale, this is rarely the case; changes to temperature, pressure, reaction time, concentration and even the reagents used may be necessary.
The manufacture of a batch of 500g of high purity retinol is a good example of this, as pure retinol is not an easy molecule to handle – it is unstable at the reaction temperature and sensitive to both air and light. Reproducibility is poor in batch mode, yields are low (typically around 40%) and the batch process takes 16 hours to complete. But perhaps the biggest difficulty in making retinol in highly pure form is that it is not possible to purify the crude product. This poses real problems for process development.
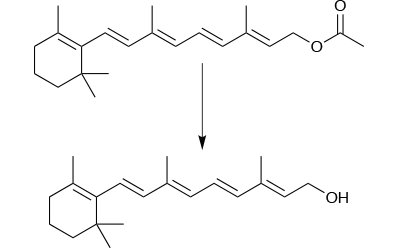
The continuous flow process was successful here (Figure 2). A key factor in this success was avoiding precipitation that blocked the reactor tube. To do this, the solvent system was changed from methanol and water used in batch mode; two separate reagent streams were fed into the flow reactor, one with 1M retinyl acetate in THF and the second 2M aqueous KOH, both at the reaction temperature of 60°C. The immediate quench flowing out of the flow reactor and into the work-up flow reactor ensured constant reaction time and conditions throughout the process.
This process has now been optimised in terms of KOH excess and flow rate; it can produce retinol in 99.99% purity with a productivity of 35g/h. This high purity can cause its own problems by crystallising out spontaneously during the continuous workup.
Higher purities
Purity issues can also be addressed through the use of a continuous flow process. For instance, ionic liquids are increasingly in demand, with applications ranging from solvents to electrolytes. However, it can be challenging to make them in sufficiently pure form for practical use, particularly at a large scale, because of the difficulty of removing impurities and solvents.
In the course of developing a range of high purity ionic liquids at a 5kg scale, there was a real problem with a yellow coloration appearing in the liquid that could not be removed if the reaction temperature rose above 20°C. Avoiding this in batch mode was problematic because the alkylation reaction is strongly exothermic and achieving purity in excess of 96% is difficult (Figure 3).
This was overcome by moving to flow; a productivity of 1.5kg/h is now possible, with purity in excess of 98%. Using a glass vessel was essential to avoid corrosion and the heat removal provided by a 2mm channel glass reactor was sufficient to prevent the coloured impurity from forming. Fast, exothermic reactions like this become manageable in flow because of the ability to remove excess heat as soon as it is generated, reducing the tendency to form byproducts.
Improved safety
Perhaps one of the biggest advantages of using a continuous flow reactor over a batch reactor is that it can improve safety when working with hazardous substances. In batch reactors, the large quantities of hazardous reagents can pose real risks to both the operator and the environment. In flow, the quantity of hazardous reagents in the reactor at any one time is much smaller and poses less of a risk.
DAST, or (diethylamino) sulfur trifluoride, is a common fluorinating reagent used in the manufacture of many key pharmaceutical intermediates that contain fluorine atoms. However, DAST is difficult to handle in batch mode because of its low heat of decomposition. While it must be purified by distillation, impurities form during the distillation that reduces its thermal stability still further. This poses an additional hazard because it reacts readily with water, forming toxic hydrofluoric acid, (Figure 4).
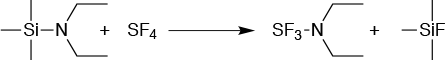
The answer lies in making DAST in situ in a flow reactor and then introducing it into a second continuous flow reactor where the fluorination itself takes place. Careful design is required since the sulfur tetrafluoride used to make DAST boils at -40°C; a way of using this as a gas in flow had to be developed. To do this, the reaction to make the DAST is carried out without solvent.
Using a continuous flow reactor, a productivity of 10kg/day was achieved. The stability of the crude product is unchanged, but the small volumes and footprint have significantly enhanced safety, and this method can be used to carry out large-scale continuous fluorinations at a lower cost using crude DAST.
Expanded chemical space
In certain cases, flow reactors enable access to new chemical entities that were previously not possible with batch techniques. If the production involves overly hazardous chemicals or simply does not work on scale-up, the small quantities in a flow reactor operating under continuous flow conditions also prove preferable to the batch reactor.
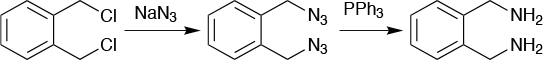
An example (Figure 5) of this is o-xylylenediamine hydrochloride. The product was needed at a kg scale as a building block, but no safe synthetic route could be found to make it in larger quantity in a batch reactor. It could, however, be made on a micro scale in batch, which helped establish the reaction parameters and solubilities, as well as checked the safety of the process. This was important, as one of the reagents used in the process is sodium azide, which is both explosive and highly toxic. When the process was moved over to a continuous flow reactor, the two steps of the reaction were separated. The formation of a diazide and the reduction to a diamine were run in separate flow reactors to develop a safe and effective process to produce an otherwise inaccessible molecule.
This process avoided the need to isolate the unstable diazide intermediate, with the crude reaction mixture flowing into the second flow reactor along with the reducing agent triphenylphosphine in toluene. The first reaction, the formation of the diazide, could safely be run at 90°C in water with a residence time in the flow reactor of 10 minutes. The reduction took eight minutes in the second flow reactor and had an overall yield of 60%. It then became possible to make 1kg/day of the diamine compound.
Unstable products
Sometimes it can be the instability of the product itself that renders it inaccessible via large-scale batch reactions. What can be synthesised on a lab or even pilot scale sometimes simply cannot be made at a large enough scale to meet commercial needs. Making many smaller batches allows demand to be met, but this is hardly efficient in terms of cost, time or capacity. Continuous flow reactors can enable products like this to be made in larger amounts by simply leaving the reactor running longer.
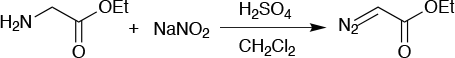
Ethyl diazoacetate (Figure 6) has many applications as a reagent in organic synthesis and is particularly useful as a carbine precursor in the cyclopropanation of alkenes. The chemical is too unstable for transport and safe use, so it must be kept in a suitable solvent. The market demand is significant, but thanks to its poor thermal stability there is a safe limit of about 3kg/batch, or a 50L reactor scale. This is nowhere near enough to meet commercial demand.
Simply by moving to a continuous flow reactor, the required scale-up to meet demand became possible. Using similar chemistry to the batch procedure in a 50ml flow reactor, the flow process gives ethyl diazoacetate as 15wt% solution in toluene, which is suitable for both transport and safe use. The capacity is currently 10kg/day of pure ethyl diazoacetate, and an expansion in capacity is planned to meet demand.
Faster AND more cost-effective
Continuous flow reactors can also offer faster and more cost-effective production, such as in the case of a patented photochromic dye that is used in a new form of colour-changing glass that changes colour much more quickly than coatings containing existing dyes. The problem with the batch process is that a very high temperature is needed – 245°C – and on scale-up the product tends to decompose. While an Indian contract manufacturer had succeeded in producing large quantities of the material, it was with a batch size limit of about 100g of product and 538 batches were required to make sufficient material. This is clearly not ideal, as the customer required 75kg of this intermediate for the fourth step of a nine-step synthesis (Figure 7).
In order to move to continuous flow, a suitable high-boiling solvent was identified from a group of glycol ethers and the process was carried out in a stainless steel tube reactor. The optimum residence time was 8 minutes with a reaction temperature of 270°C. By running the flow reactor 24/7, sufficient material for three years of the customers’ production was made in two and a half weeks. Manufacturing cost was reduced to 12% of the batch process; the defined and short reaction times enabled cost-efficient production to be achieved.
In closing
There are many areas where continuous flow reactors have proven advantages, but it must be emphasised that there are processes that currently cannot be performed in continuous flow reactors or will not be beneficial, compared with batch reactors. One example is heterogeneous reactions where the small tubes of flow reactors ranging from 10 um to mm have greater potential of becoming clogged.
Flow reactors are an additional and complementary manufacturing toolkit for the fine chemical and pharmaceutical industry as they enable safer and more cost-efficient production for many types of reactions. There is no doubt that with the increasing number of chemists using flow reactors and developing continuous processes, we will see many new exciting products which are difficult to make and scale in a batch process.
Andreas Weiler is head of strategic marketing; Gregor Wille is senior scientist; Patrick Kaiser is principal scientist and Stefan Gladow is manager, PRD at SAFC




