5 Mar 2014
Prof Matthew Gaunt began his talk on Catalytic C-H bond functionalization - a new paradigm for molecular synthesis on 27 Feb 2014 by relating synthesis reactions using catalysis to the global economy. He stated these reactions contributed to 35% of it. This contribution ranged from fuel and energy to pharmaceuticals and food.
Prof Gaunt then went on to talk about catalysts used in organic chemistry. He said the chemical reactivity of an organic substance can be exploited by using a transition metal catalyst. These organometallic catalysts can steer a reaction via one of three routes resembling carbocations, carbanions or carbon radicals. If the metal atom possesses a charge or ligands, the metal's characteristics (acting as an electrophile, nucleophile or neutrally) can change resulting in a different form of the carbon intermediate.
Transition metals are very important catalysts in chemical synthesis but they can be extremely expensive (palladium (Pd), $23470/kg, ruthenium (Ru), $16500/kg, and rhodium (Rh), $27550/kg). Despite the cost, palladium is used as a catalyst in the Suzuki coupling reaction (reaction which joins aromatic groups together and which earned the Nobel Prize in Chemistry in 2010); much less than 1g (0.0001 mol %) of Pd is used, but this can produce tonnes of product.
The Suzuki reaction requires pre-installed functionality on the reactants (for example a halogen and a boronic acid group) to which the palladium catalyst can bond. It would be more desirable if there was some way to activate the C-H bond without the need for a pre-installed functionality. If this could be achieved, the synthesis would be more efficient with fewer steps and waste and cost would be reduced.
One of the main problems that scuppers an easy solution is the lack of reactivity of the C-H bond. The C-H bond is comparatively strong; it requires about 420kJ/mol to break compared with roughly 350kJ/mol for a C-Cl bond. Also, a C-H bond is not very polar due to the similar electronegativities of C and H (2.55 and 2.20 respectively) whereas a C-Cl bond is polar due to its larger difference in electronegativities between C and Cl (2.55 and 3.16 respectively).
This electronegativity difference, however, allows for the insertion of the transition metal between a C-Cl bond as shown in many palladium catalysed reactions. Palladium can also form a complex with a hetero atom and activate a nearby C-H bond. The resulting organometallic complex can then react with a C-H bond in the same molecule as shown in the scheme or another molecule that also binds as a ligand.
It is also very difficult to select for a specific C-H bond as this is the most ubiquitous bond in organic chemistry. However this might be overcome by using the proximity of heteroatoms to select for a C-H bond, for example using heterocyclic aromatic rings with nitrogen as shown below.
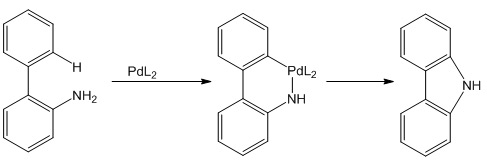
The use of these organometallic intermediates formed will revolutionise chemical synthesis forever.
A cheaper alternative to Pd (II) - catalysts are Cu (III) - catalysts. Pd2+ is isoelectronic with Cu3+ as they both have eight electrons in their d-orbitals which implies they have similar reactive properties. When the Cu (III) catalyst was tested with biaryls (two aromatic groups bonded together), the resultant species behaved by bonding other functional groups to the meta position of the aromatic rings, like an electron withdrawing group bonded to an aromatic ring would.
This was most unusual as aromatic rings are electron donating and should be para directing. Currently, the mechanism of the reaction is unknown, but the implication for synthetic organic chemistry huge. Due to this new type of molecule, functional groups could be directed to positions on aromatic rings which would usually not be accessible.
This has great significance for the manufacture of pharmaceuticals as it could lead to old drugs being 'resurrected'. Many drugs whose structure contains multiple amines bonded to biaryl compounds have not passed clinical trials as their toxicity is too great. Using Cu (III) catalysts, these amine groups could be directed to a different position on the biaryls which may reduce their toxicity.
Prof Gaunt gave a fascinating and enlightening talk about using catalysts to activate the C-H bond in organic chemistry; a relevant research area that is likely to have an impact in the pharmaceutical sphere in the near future. The Society thanked him for his engaging lecture.
Natasha Jewa, James Nugent
Charterhouse
