Whether due to changes in policy or consumption of available fossil fuels, the world will require alternative sources of energy, especially given rising global energy demand. However, one of the main factors limiting the widespread use of renewable energy, such as wind, solar, wave or geothermal, is our inability to store the energy produced by these intermittent sources.
Storage of energy from carbon-neutral sources, such as electricity from solar or wind, can be accomplished through many routes. One approach is to store energy in the chemical bonds of fuels. The conversion of low-energy compounds, such as water and carbon dioxide, to higher energy molecules, such as hydrogen or carbon-based fuels, enables the storage of carbon-neutral energy on a very large scale. While the large difference between the low and high energy states is attractive for energy storage, it presents challenges for the required chemical transformations.
Carbon dioxide is a molecule with many ties, as most carbon-based materials are derived from, or lead to, carbon dioxide. The combustion of fossil fuels produces about 30bn t/year of carbon dioxide. The CO2 resulting from fossil fuels could be captured, but it would need to be stored indefinitely to avoid contributing to global climate change. Alternatively, captured CO2 could be converted to fuels as a means to store carbon-neutral energy. While the CO2 stored in such ‘carbon neutral’ fuels will enter the atmosphere when the fuels are consumed, they will result in a net decrease in the overall emissions of CO2 to the extent that they are used in place of fossil fuels.
Ultimately, a net-zero carbon emission cycle will require capturing CO2 from the atmosphere and converting it to a fuel. The energetic cost of capturing CO2 from air could be quite small, as the entropic difference in energy between the current concentration of CO2 in the atmosphere and pure CO2 (at 1 atm) is only 19.5kJ/mol. However, the capture of CO2 from air with high energy efficiency will require the development of new chemical approaches, rather than relying on the highly favorable, and thereby energy inefficient, reaction of CO2 with hydroxides. Furthermore, accurate cost estimates for the capture of CO2 from air remain challenging, as the design of contactors or reactors will depend on the specific chemistry developed.
While capture and conversion of CO2 from air provide challenges, nature demonstrates that this process is feasible. Plants use CO2 from air as the primary source of carbon to enable growth and propagation, making CO2 capture merely a means to accomplish a larger goal. Similarly, designing a synthetic carbon-neutral, carbon-based fuel cycle could enable renewable energy storage in the form of transportable, high energy density liquid fuels by exploiting ambient CO2.
The ability to generate liquid fuels from CO2 is the most significant advantage, relative to hydrogen, as a fuel. Carbon-based fuels can have many forms, ranging from relatively simple one-carbon fuels to complex multi-carbon fuels. Of the one-carbon fuels, methanol has the highest volumetric energy density under ambient conditions, as it is a liquid and contains the energy of three equivalents of hydrogen. Due to its simplicity, compared with multicarbon fuels, methanol is a target for many research programmes focused on the reduction of CO2.
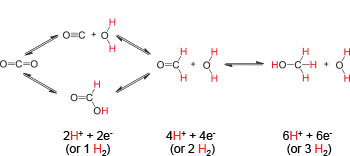
Scheme 1
Catalytic reduction
Catalytic reduction of CO2 using carbon-neutral energy, such as solar energy, can occur by photocatalysis, electrocatalysis, or catalytic hydrogenation. In photocatalysis, solar energy is used directly, whereas in electrocatalysis or hydrogenation, solar energy is first converted to electrical energy or H2 and then used for the reduction of CO2. Each approach has advantages and disadvantages, but in principle, any of these approaches could be used (Figure 1).
Direct utilisation of solar energy through photocatalysis has the prospect of higher energy efficiency and lower costs through integration, however, dispersion of the catalysts over the entire photocollector means that the produced fuels will also be dispersed. Conversely, use of photovoltaic devices to convert solar energy to electricity followed by electrocatalysis may result in lower efficiency and higher costs due to the use of separate devices; however, the fuel production can be localised. Additionally, localisation of the catalyst would simplify its replacement or regeneration, compared with systems in which it is dispersed over, or integrated with, a photocollector.
Hydrogenation of CO2 would require the production of H2 by photocatalysis or electrocatalysis followed by a separate hydrogenation reactor. This increased number of steps could mean higher costs; however, due to the number of variables involved in each approach, the ‘best’ solution is currently hard to predict.
For any of these transformations, the overall reaction is the reduction of CO2 and the oxidation of water. While the oxidation of water does not necessarily seem connected, it is an essential component of fuel production. The reduction of CO2 has to be coupled to an oxidation reaction, and the oxidation of water to produce O2 is the reverse of fuel utilisation reactions, thereby closing the cycle. Moreover, on the scale needed for fuel production, O2 is the only desirable oxidation product.
In comparing the reduction of protons to form H2 and the reduction of CO2, the energy required per reduction equivalent is quite similar. The overall energy requirement is really due to the need for both the fuel forming – reduction – reaction and the water oxidation reaction. Therefore, the thermodynamic energy requirements for reducing CO2 through photocatalysis, electrocatalysis, or catalytic hydrogenation are all similar, especially given that hydrogenation of CO2 will require a renewable source of H2.
Catalysts for the reduction of CO2 have many forms, from heterogeneous catalysts, such as copper and copper oxides, to molecular catalysts, which are often metal complexes but can also be metal-free catalyst systems. Inexpensive catalysts are required due to the global scale at which energy conversion technologies are needed. First row transition metals are cheaper, but typically are less effective catalysts than their precious metal counterparts. Understanding how to rationally design inexpensive catalysts for the inter-conversion of energy and fuels would be a remarkable advancement towards storage for renewable energy.
Conversion of CO2
The initial steps in the reduction of CO2 involve either formation of C-H bonds, such as the reduction of CO2 to formate, or the cleavage of C-O bonds, such as the reduction of CO2 to CO, as illustrated in Scheme 1. Catalysing each pathway is challenging, even though both involve relatively simple bond-forming and bond-breaking reactions. Understanding the energetics for these simple steps can greatly aid in the design of catalysts for these transformations, as the bond strengths of the catalytic intermediates should be energetically matched to these simple bond-forming and bond-breaking reactions. Balancing the energetics can yield improved catalytic rates and energy efficiencies.
Transition metal complexes capable of catalysing the hydrogenation of CO2 undergo reaction of a metal-hydride with CO2 to give the metal complex and formate. This process is sometimes performed electrochemically using protons and electrons for reduction rather than H2, but it is more often a thermal hydrogenation process. Remarkable new catalysts from numerous research groups have been investigated for this transformation, and great progress is being made toward the development of non-precious metal catalysts.
For CO2 hydrogenation, two primary challenges remain: achieving and exceeding the performance attained with precious metals while using inexpensive metals, and reducing CO2 to higher energy density products than formate. The performance of catalysts using non-precious metals is often lower in terms of rates, energy efficiencies, and lifetimes, however, there is not an inherent limitation preventing non-precious metal catalysts from achieving comparable performance.
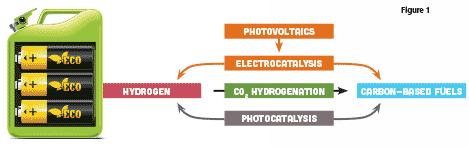
Figure 1
In the second challenge, the volumetric energy density of formic acid is approximately half of that for methanol, which in turn is approximately half of that for petroleum. Increasing the energy density of fuels produced from CO2 will require reduction past formate/formic acid, a transformation for which very few molecular catalysts are known.
Molecular catalysts for the reduction of CO2 to CO have also been explored extensively, but unlike the reduction of CO2 to formate, molecular catalysts for reduction of CO2 to CO typically operate electrochemically rather than thermally. As with the generation of formate, substantial advances have been made in catalysis without precious metals; however, the performance of catalysts still needs to be improved. In particular, the non-precious metal catalysts require higher performance: milder potentials, higher catalytic rates, and longer lifetimes, although the latter can be a challenge to measure for homogeneous electrocatalysts with reasonable stability.
Comparing the lifetimes of molecular electrocatalysts can be greatly aided by confinement at the surface of an electrode. Catalyst lifetimes are often reported as turnover numbers, which are usually measured with controlled potential electrolysis experiments. For homogeneous catalysts, these measurements provide the average number of turnovers for all of the catalyst in solution, however, the catalyst is only active while near the electrode surface. The bulk catalyst in solution is not active but is counted in the average number of turnovers, and therefore the reported turnover numbers are artificially low. By confining molecular catalysts to electrode surfaces, this averaging can be avoided and meaningful catalyst lifetimes can be measured.
Beyond CO2 reduction, a longstanding challenge for molecular catalysis is the reduction of CO. Extensive work has been performed on the first steps of this reduction, but actual catalysts are limited in number. The increasing use of multifunctional interactions in catalyst design, a theme that is ubiquitous in nature, will likely accelerate the development of catalysts for both the reduction of CO2 and the subsequent reduction of CO. Understanding the energetics for each potential catalytic intermediate for CO reduction will help in the design of functional catalysts.
Synthetic carbon cycle
The development of a synthetic carbon cycle for the purpose of energy storage will enable increased use of renewable energy. The production and use of carbon-neutral, carbon-based fuels is inspired by nature through the use of atmospheric CO2 as a resource for a larger purpose. While the timeframe for implementation of carbon-neutral, carbon-based fuels will depend upon many variables, it is clear that this will require fundamental advances in the underlying science.
Our research group at Pacific Northwest National Laboratory in Richland, US, is focused on the reduction of CO2 using molecular catalysts that contain abundant metals. As inspired by nature, our aim is to develop an understanding of how to design catalysts by using multifunctional interactions, while concentrating on the energetics of the required bond-forming and bond-breaking reactions. For the reduction of CO2 and the subsequent intermediates, we are pursuing electrocatalysts as well as hydrogenation catalysts.
In addition to these fuel production reactions, we are interested in designing catalysts for the reverse reactions for fuel utilisation. The use of fuel cells will enable higher energy efficiencies, but will require effective electrocatalysts for the targeted fuels. We seek to understand how to rationally design catalysts for each of the chemical steps in a complete fuel production and utilisation cycle.
Aaron M. Appel is a senior scientist in the Catalysis Science Group at Pacific Northwest National Laboratory, Richland, Washington, US
Further reading
1 T. R. Cook et al, Chem Rev., 2010, 110, 6474.
2 G. A. Olah, G. K. S. Prakash, and A. Goeppert, J. Am. Chem. Soc., 2011, 133, 12881.
3 A. M. Appel et al, Chem. Rev. 2013, 113, 6621.
4 C. Costentin, M. Robert, and J.-M. Savéant, Chem. Soc. Rev., 2013, 42, 2423.
5 Y. Oh and X. Hu, Chem. Soc. Rev., 2013, 42, 2253.
6 E. V. Kondratenko et al, Energy Environ. Sci., 2013, 6, 3112.
The author’s work in this area is supported by the US Department of Energy Basic Energy Sciences, Division of Chemical Sciences, Geosciences & Biosciences. Pacific Northwest National Laboratory is operated by Battelle for the US Department of Energy.





