Modern techniques for analysing tiny quantities of metals in the brain are yielding insights into the diseases of ageing such as Alzheimer’s and Parkinson’s disorders, says Joanna Collingwood
The biochemistry of the human brain inevitably includes many elements, including several metals, required throughout the body for normal maintenance and function. As well as sodium, potassium and magnesium, for example, brain tissue contains smaller amounts of iron, zinc and copper, along with even smaller quantities of ‘trace’ metals like molybdenum, vanadium, chromium, cobalt and nickel. Redox active metals, such as iron and copper, typically play important roles, for example, in building healthy brain cells, enabling communication between neurones, and protecting against excess free radicals. But their faulty metabolism may also contribute to much of the oxidative stress implicated in the degeneration of nerve cells or neurons linked to many of the common diseases of ageing, including Parkinson’s, Alzheimer’s and Huntington’s diseases, and several rare disorders classed as ‘neurodegeneration with brain iron accumulation’ (NBIA).1
Metal biochemistry of the brain
Understanding the metal biochemistry of the brain could potentially shed important insights into the nature and progression of these devastating diseases. But how do we know which metals are present, and how much we can learn about them? Traditionally, investigations of metals in the brain have been made in post-mortem tissues, stained to produce contrast that will reveal the metal ions of interest. However, some metals are easier to ‘see’ than others, and staining techniques may be non-specific for metal ions with similar properties. Such methods may also be near-impossible for soluble ions, particularly if their distribution in the tissue needs to be preserved – unless a reagent can be used to produce an insoluble salt containing the ion of interest. While some stains are of great value in indicating metal ion distributions at cellular and regional levels, variables in tissue preservation, staining protocols and lack of specificity or sensitivity to certain metal ions can make this approach less than ideal for characterising metal ions in brain tissue.
Metal ion concentrations, including those present in quantities far too low to be revealed with staining, have been quantified in brain tissues by many research groups using sensitive elemental analysis techniques. Donated brain tissue, from biopsy or autopsy, is digested to produce a solution in which the concentrations of each metal can then be measured, for example, by mass spectrometry. But while this is an accurate way of quantifying metals in tissues, it means that any detailed information about the spatial distribution of the metal ions in the tissue, and the form in which they are bound, is lost. While alternative approaches, including electron probe microanalysis, laser microprobe emission spectroscopy, and Mössbauer spectroscopy, yield valuable information about the distribution, oxidation state, and the local environment of the metal ion in autopsy tissues, all have limitations, being semi-quantitative, destructive and/or limited in spatial resolution, sensitivity and specificity.
My research group at the University of Warwick, and our collaborators at the University of Keele, UK, and University of Florida, US, are among several using newer advanced techniques for visualising and characterising metal ions in tissues. We are using micro-focused beams of X-rays at synchrotron facilities to map the fluorescence from metal ions in tissues and to capture the absorption spectrum from specific sites of interest. The microfocus synchrotron beam provides us with an ultra-sensitive high-spatial resolution technique to map and characterise metal ions in tissues, and the wavelengths we use are particularly suitable for detecting transition metal ions. It has the added advantage that the tissue requires minimal handling, no contrast agents, and the technique is non-destructive.
I first became interested in iron biochemistry as a PhD student, after hearing a seminar about work at Jon Dobson’s lab at Keele. Dobson has a particular interest in the potential role of nanoscale iron oxides in neurodegenerative disorders, including the mixed valence iron oxide magnetite, which has distinct magnetic properties compared with the usual ferrihydrite-like cores found in ferritin. He uses a range of techniques, including SQUID magnetometry, to analyse the magnetic properties of iron in the brain.
I joined the group as a postdoctoral research fellow in 2003, supported by the Alzheimer’s Society, and was involved in the Keele-Florida collaboration’s first synchrotron work on human brain tissue where we demonstrated the presence of magnetite [Fe3O4] in a case of Alzheimer’s disease.2 At that point we were using the micro-focusing capabilities developed at the Materials Research Collaborative Access Team (MR-CAT) beamline at the Advanced Photon Source (APS) in Chicago, US, a development involving Mark Davidson at the University of Florida.
The group has been using the Diamond synchrotron X-ray facility at Harwell in Oxfordshire since October 2007 when the I18 microfocus beamline became available to general users. Access to the Diamond facility is through a competitive proposal system in order to obtain the required ‘beamtime’. In our first experiment, we started by comparing samples we had already measured at the APS synchrotron in Chicago, and found that the results were in good agreement. Since then, the main focus has been two parallel studies: an EPSRC-funded project to look at iron in the Parkinson’s disease brain, and a US National Institutes of Health (NIH)-funded project – with the University of Florida – to look at iron in the Alzheimer’s brain.
In a typical experiment, we map and characterise a set of tissue sections donated by patients with these disorders, and from donors of the same age without a neuropathological diagnosis. The iron analysis is performed in order to determine the iron distribution and form in the tissues; unlike with staining, the mapping detects iron regardless of how it is bound, and the absorption spectroscopy allows ‘fingerprinting’ at chosen sites for iron bound in a variety of forms. With this kind of work it is necessary to look at multiple cases, to determine the spectrum of variability in a given disorder.
We are focusing attention on regions of the brain that are particularly vulnerable to neurodegeneration. In Parkinson’s disease, our investigation includes the substantia nigra, which contains the dopaminergic neurones that play an essential role in controlling movement. In an earlier study led by Chris Morris and colleagues at the University of Newcastle, UK, we showed – using an electron microprobe technique – that the concentration of iron in Parkinson’s dopaminergic neurones is significantly higher than in healthy control brain tissue,3 and discussed some of the mechanisms that might be responsible. The synchrotron analysis is an extension of this work: it allows us to compare the chemical state of the iron to see if we have ‘more of the same’, or altered bound forms of iron, and the synchrotron mapping technique contains sensitive and specific information for several key transition metals, allowing us to simultaneously determine distributions for Fe, Cu, Zn, Mn, and any other metals of interest that are within range, at cellular or sub-cellular resolution.
Study of brain tissues
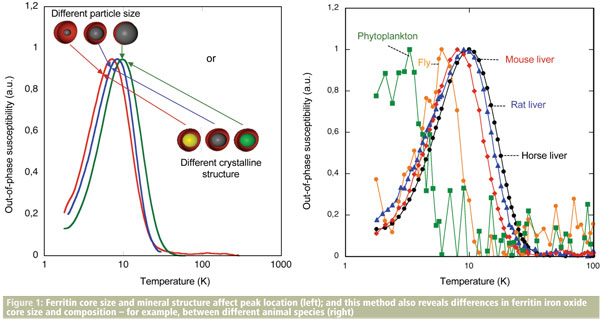
We use a variety of tools in our study of brain tissues, including electron microscopy, SQUID magnetometry, and now also magnetic resonance imaging.4 Our findings form part of a large and incomplete jigsaw, as each technique can only reveal part of the story. For example, if we want to determine whether two samples have differing concentrations of iron, this can be done by mass spectrometry. A protein assay could tell us if the amount of iron storage protein ferritin differed between the samples, but what if the iron oxide content of the ferritin differs between the two cases? Electron beam techniques can determine ferritin core sizes, and electron and X-ray beam techniques allow differences in the type of iron oxide to be observed, but the latter either require extraction of the ferritin or are site-specific: they do not facilitate quantitative analysis of ferritin cores in bulk tissue samples. For this, magnetometry is useful: isothermal remanent magnetisation is one of the techniques to analyse iron oxide nanoparticles in biological tissue. A subtle approach exploiting this technique has been developed to look at properties of ferritin cores by Francisco Lázaro and his colleagues at University of Zaragoza in Spain as in Figure 1.
An important aspect in this kind of work is sample preparation. When looking for common metal elements at sub-ppm concentrations, it is important to minimise opportunities for contamination. With this in mind, we take precautions to preserve integrity – by including tissues that have not been chemically fixed, sectioning with sapphire blades as opposed to steel, using high purity microscope slides rather than regular glass, and so forth. The samples are provided by tissue banks, following ethical approval for the study from a UK ethics committee, and approval from the tissue bank itself. Brain tissues in the bank are donated with the informed consent of participants. So where is the evidence to suggest that metals play a role in neurodegenerative brain diseases? The research literature is extensive, ranging from molecular-level studies to clinical observations, and while the picture is far from simple, there are some strong common threads.5 In particular, the evidence for metal-ion-mediated oxidative stress in neurodegeneration is compelling, although the exact mechanisms remain unclear. There are various hypotheses about the way in which metal ions may contribute to the disease process, but the evidence stems from association, and from in vitro and animal model testing of hypotheses in most cases. The formation of pathologic protein aggregates is a feature of many neurodegenerative disorders – for example, amyloid beta in Alzheimer’s plaques, and alpha synuclein in the Lewy bodies in Parkinson’s disease – and the impact of various metal ions on the aggregation process is an active area of research.
In a recent study of protein aggregation behaviour led by Chris Exley’s group at Keele, for example, we saw that copper had the capacity to alter the betapleated sheet structure found in Alzheimer’s plaques and to form spherical protein structures referred to in the literature as spherulites. We observed a spherulite structure with near-identical properties in Alzheimer’s tissue, and used the synchrotron mapping technique to confirm the association of copper with these structures in the tissue.6
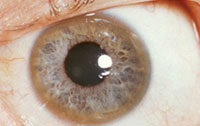
Direct and specific observation of metal ions in the brains of living patients remains a formidable challenge. There are disorders in which significant changes in concentrations of transition metal ions can be used as a diagnostic marker, such as Wilson’s disease where copper overload becomes evident as ‘Kayser-Fleischer’ rings in the eyes, but this is the exception rather than the rule. The use of MRI to measure iron overload in the liver has been explored by several groups, including by Tim St Pierre and colleagues at the University of Western Australia, and we are interested to know if similar approaches can be used to quantify iron changes in the brain – an idea that has been around since the development of MRI, although there are some challenges still to be overcome.
George Bartzokis and coworkers at UCLA, California, US, for example, have over the past decade developed and applied an MRI approach that involves scanning study participants at two different magnetic field strengths to estimate ferritin concentration in regions throughout the brain. Using this approach, the group observed various differences between patients with neurodegenerative disorders and healthy age-matched participants, including evidence of increased ferritin concentration in vulnerable brain regions for a range of neurodegenerative disorders.
When cases of iron overload are diagnosed in the body, for example, the liver iron overload disorders, it is currently possible to treat these with chelating drugs. It is not yet clear, however, to what extent this approach could be used as a clinical therapy for neurodegenerative disorders, where any changes may be highly localised. In some regions it may be an iron deficiency, or deficiency stemming from faulty metabolism, that occurs. As research continues in these areas, even the most accepted models of metal ion uptake, transport and utilisation in the brain are open to question.
Neurodegenerative disorders are among the most challenging and distressing diseases, and metalloneurochemistry and related disciplines could provide insight and therapeutic opportunities. If in parallel we can improve detection of associated metal ion changes in the brain, then – regardless of whether these disease-related changes are cause or consequence – perhaps we can increase the window of opportunity for intervention.
Joanna Collingwood is assistant professor in the biomedical and biological systems laboratory in the School of Engineering at the University of Warwick, UK.
References
1. Crompton, D. E., et al., Blood Cells Mol Dis, 2002, 29, (3), 522.
2. Collingwood, J. F., et al., J. Alzh. Dis., 2005, 7, (4), 267.
3. Oakley, A. E., et al., Neurology, 2007, 68, (21), 1820.
4. Collingwood, J. F., et al., Movement Disorders, 2008. 23,(1), S62.
5. Crichton, R. R. and R.J. Ward, Metal-based neurodegeneration: from molecular mechanisms to therapeutic strategies. Chichester, Hoboken, NJ: J. Wiley & Sons, 2006.
6. Exley, C., et al., J. Alzh. Dis., accepted.
7. Collingwood, J. F., et al., J Alzh. Dis., 2008, 14, (2), 235.





