Water purity is an increasingly important issue for medicinal chemists seeking to minimise potential sources of contamination in the lab, writes Julie Akana
Laboratory water has multiple uses in the life sciences sector, from glassware rinsing to highly sensitive applications involving biological samples. Theoretically, pure water consists only of water molecules (H2O) and contains no impurities. In practice, however, it dissolves and suspends a number of common contaminants, such as trace organics, particles, bacteria, fever inducing pyrogens, and importantly, nucleases, all of which can have a detrimental effect on the resulting data.
Defining standards
While industry standards for pure water have not changed dramatically over the past few decades, water purification technologies are undergoing continuous refinement, with point-of-use benchtop systems now making ‘ultrapure’ water routinely accessible.
Increasingly stringent quality control measures enforced within many laboratories make methods to reduce contamination critical at every step of the drug discovery and development process. Since water is so abundant in the lab, and in everyday life, it can often be overlooked as the potential source of a problem. As awareness has improved, the demand for ultrapure water systems has increased. And, so too, as analytical techniques have become more sensitive, the requirement to significantly reduce, if not eliminate, contaminating substances has grown.
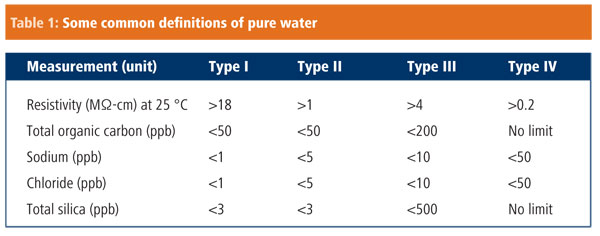
Because water purity is so important, several professional organisations have established water quality standards. These include the American Society of Testing and Materials (ASTM) and the Clinical and Laboratory Standards Institute-Clinical Laboratory Reagent Water (CLSI-CLRW). These organisations have similar, but not identical, definitions for highly purified water, which are determined on the basis of parameters such as conductance, resistance, the presence of colloids, bacterial count, organic content and pH. The most commonly used standard, ASTM D1193-6 is summarised in Table 1. Using ASTM nomenclature, type IV is the lowest grade of purity, while type I is the highest grade, suitable for highly sensitive applications. These can then be further subdivided into A, B and C, where A is the most pure, as a measure of heterotrophic bacteria count (CFU/ml) and endotoxins (units/ml).

Application critical
Highly critical analytical and life science applications, such as high performance liquid chromatography (HPLC), polymerase chain reaction (PCR) and electrophoresis, for example, will suffer the most from high impurity levels. In essence, the presence of such impurities will have a significant adverse effect on resulting data, rendering the experiment void. For example, if nucleases are present in a PCR reaction, they will effectively cleave any DNA, including the primers and probes. Amplification will therefore not occur.
As the techniques employed for these sensitive applications have advanced, users are able to detect increasingly minute quantities of various products. However, in detecting these small volumes, instrumentation is now also able to detect trace impurities as well, which will skew the obtained data. As a result, the water used must be as close as possible to theoretically pure.
Obtaining ultrapure water

Traditional methods for ensuring that water is nuclease-free include the use of a 0.1% solution of diethyl pyrocarbonate (DEPC), which effectively inactivates ribonuclease enzymes (RNases) in buffers and water. However, since DEPC is a non-specific inhibitor, it must be removed from treated solutions before being used with nucleic acids. This method is therefore time-consuming and can also be toxic. More recent developments have included efforts to increase efficiency through improvements in filtration and separation technologies, as well as decreasing operational costs.
A point-of-use ultrapure water system that can produce nuclease-free water without DEPC treatment would therefore be a valuable piece of laboratory equipment, eliminating a lengthy and potentially toxic method. Such water systems use both UV and UF technology, successfully combining the advantages of each to provide ultrapure water.
The benefits of UV: Ultraviolet light can effectively eliminate trace organic compounds and inactivates microorganisms through its photochemical oxidative action, which essentially oxidises organic compounds within the sample to produce carbon dioxide. Through the use of UV light at both 185 and 254 nm, very low bacteria and total organic carbon (TOC) levels can be obtained. The light produced at 254nm has optimal antibacterial properties, causing DNA mutations, whereas light at 185nm has the ability to break organic bonds to generate free radicals, which subsequently causes cell death. This combination of light therefore allows for total oxidisable carbon levels of less than 5 ppb.
The benefits of UF: Ultrafiltration uses size exclusion processes to remove particles and macromolecules such as pyrogens and nucleases. They are captured on the hollow polysulfone fibers of the filter membrane and flushed out of the system via a reject stream. These filters are positioned at the end of the system to ensure the elimination of all nucleases and pyrogens.
One such system, which combines the advantages of the UV and UF technologies, is the Thermo Scientific Barnstead Nanopure Life Science (UV/UF) system, which produces ultrapure water with RNase and DNase levels below the detectable levels, at 0.003 ng/ml and 0.4 pg/μl, respectively.
The three Rs of recycling
Primarily designed to produce pure water, manufacturers of ultrapure systems also need to take into account a host of additional issues, including global water shortages and recycling issues. In order to remain environmentallyfriendly, suppliers need to provide ways to reduce waste and water consumption, reuse cartridges and recycle. Cartridges made from high-quality ion exchange resins have an extended life-span, reducing the need for replacement along with the need to re-order ship and handle new components. Plastic cartridge casings and endcaps are designed so that the plastic and resin can be recovered, reground and reused. In addition, all systems marked with the WEEE symbol have been produced in compliance with the WEEE directive, which aims to reduce the waste arising from electrical and electronic equipment.
Recent industry trends have resulted in an increase in the use of pure water within various laboratories. It is therefore vital that different levels of purity and the techniques available to achieve them are understood. When used for highly sensitive analytical purposes, water needs to be produced that is essentially free from any impurities. As such, this ultrapure water can be produced via combined UV oxidation and ultrafiltration, which is both easy and fast.
Julie Akana is an associate product manager at Thermo Fisher Scientific in Asheville, NC, US.