
Our exploitation of the living wealth of the sea is longstanding. Seaweed use in China dates back more than 4000 years, sea snail farms – the source of Tyrian purple – were set up in the Mediterranean by the Phoenicians 3000 years ago, and nori (Porphyra) farming was taxed in Japan in 701 AD.
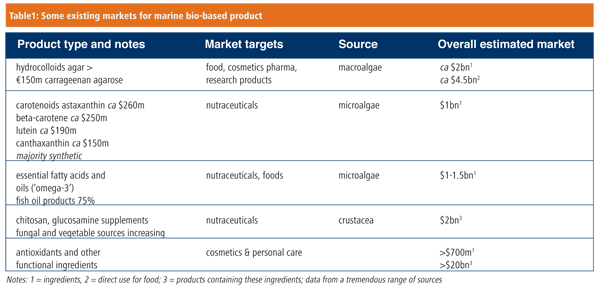
Today, several industries have emerged from our mining of the ocean’s resources, based on bioactives from marine microbes; hydrocolloids, antioxidants and essential fatty acids from microalgae and seaweeds; and biomaterials such as coral skeleton and collagen for human body repair (Table 1). In addition, there is now a new wave of more innovative products, including enzymes and platform or ‘building block’ chemicals for industrial biotechnology, as well as pharmaceutical bioactives. According to Global Industry Analysts, marine bioresources are expected to give rise to ‘marine biotechnology’ products worth over $3.75bn by 2012, particularly functional ingredients for nutritionals, cosmetics and pharmaceuticals.
Marine molecules to contribute strongly to cosmetics
The market for nutraceuticals is expected to reach almost $22bn by 2013, with antioxidant pigments (lutein and carotenoids), glucosamine, omega-3 fatty acids and minerals (calcium and magnesium) all contributing from marine sources. Marine microorganisms and algae remain an underexploited source of omega-3 fatty acids – although algal oils are added to infant formulae – minerals, probiotics, prebiotics and non-hydrocolloid functional ingredients. Marine molecules are expected to contribute strongly to cosmetics as well, because of increasing interest in antioxidants, anti-ageing and skin protection.
New anti-cancer, anti-inflammatory, antiparasitic and especially antimicrobial agents continue to be needed. One aspect of marine-derived compounds is their modes of action, often totally unrelated to terrestrial biochemistries and so able to overcome human and animal pathogen and cancer-cell defence mechanisms. Adhesive molecules for surgery, coatings and anti-biofilm molecules for medical devices, and nanostructured materials to house cells used in bioartificial organs, are other possible advances.
Marine bioprospecting has been going on for decades, since the extraction of Ara-C from sponges in 1945. It took 40 years for companies to be founded based on bioactives from the sea, the two longestsurviving being Nereus Pharma, founded in 1988 in California by private investors, and PharmaMar, which has been in business since 1986 as part of Spain’s Zeltia Group. By 2004, Nereus had a nine-compoundstrong pipeline, but five years later only three have survived, plus two new pre-clinical leads. PharmaMar is the most successful of the marine-focused pharma companies and has gained two regulatory approvals for Yondelis in the EU, for soft tissue sarcomas and ovarian cancer treatment. It has three other marinederived oncology products in late-stage development. Aquapharm, Marinomed and Glycomar are smaller and newer companies following a similar pathway to make use of marine biodiversity.
Securing supply of the ocean’s bioactives is fraught with difficulties. Those from invertebrates are usually synthesised not by the sponge, actinozoan, sea-fan or sea-squirt cells, but by symbiotic microbes, which could be bacteria, Archaea, algae or cyanobacteria, such as Synechococcus. So far, getting these endosymbionts to thrive in isolation is an uphill struggle. Even when a marine microorganism is not cell-associated, its culture may require very specialised bioreactors, to deal with hypersalinity, very high or low temperatures, or high pressures. Nautilus Biosciences Canada, for example, has a promising method for independent growth of endosymbionts that produce antioxidant pseudopterosin analogues, while Aquapharm and Croda are investigating the best methods of saline bioreactor fermentation.
Structural characterisation and synthesis removes entirely the need for wild harvesting and has been key to several important developments, such as Nereus’s NPI-0052 and NPI-2358. Aquapharm works with the University of Aberdeen in Scotland on characterising and synthesising promising extracts. PharmaMar’s sponge farms provided barely enough Yondelis for clinical studies, so a semi-synthetic production pathway was developed, starting with an intermediate from a Pseudomonas fluorescens strain.
Over all this, there is a real risk of too much novelty – of potential end-users such as the pharma industry backing away from an avalanche of new molecules that is even bigger than their existing molecule collections. Companies such as Aquapharm, LibraGen, Ktedogen and Bioalvo have amassed significant collections that include marine organisms and extracts, and are pushing to make use of them. There is a need here for high-content intelligent and predictive screens to accelerate the flow-through of novelty to useful end-points, and we see a number of companies developing these for use with their own and other people’s materials, such as Germany’s BRAIN and the European Screening Port. Aquapharm, Glycomar and Marinomed also have in-house tailored screens, focused on infection, cell physiological reactions, antivirals, inflammation and allergy.
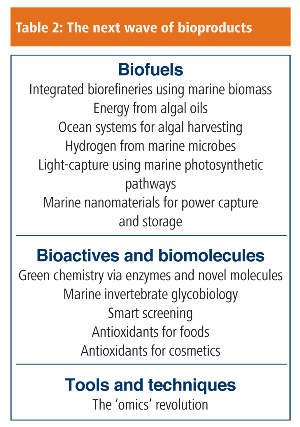
Table 2 suggests where R&D and innovation could provide the next generation of marine products. Continuing efforts in biocatalysis could make a significant impact on power usage for clothes washing, or for food and beverage processing by using extremophile enzymes – it is not difficult to imagine large-scale use of psychrophilic enzymes that work at 0–5oC rather than 25–35oC, for example.
Marine biomass for green chemistry
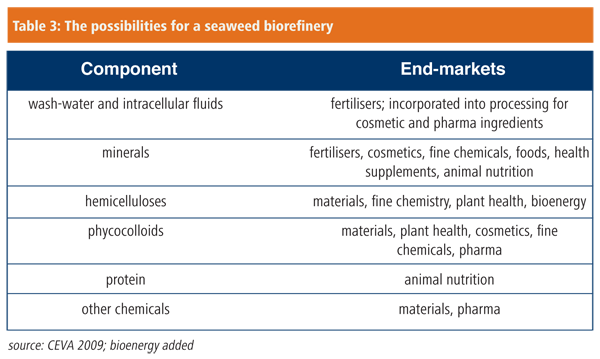
In addition to deriving new enzymes for green chemistry from marine sources, as Novacta Biosystems, Verenium and Ingenza are doing, some marine organisms provide biomass for chemical refinery processes. In the case of CEVA (Centre des Études et Valorisation des Algues) in France, the biomass is macroalgae. CEVA was founded in Brittany to do something about seaweed washed up on beaches and left to rot and stink. CEVA has a complete biorefinery approach to exploiting seaweeds, generating products that go far beyond hydrocolloids, with sales or potential sales in eight or more different market sectors (Table 3). CEVA’s biorefinery approach transforms the colloid ulvan from green seaweeds to iduronic acid, a platform chemical for the petrochemical-plastics chain, using ulvan lyase; extracts rhamnose, one of the typical seaweed sugars, for hemi-synthesis of the blood thinning agent heparin; develops biological surfactants for industrial use from oligo-alginates and oligo-ulvan; and chemically derivatises phycocolloids using conventional starch and cellulose modification techniques.
The biorefinery concept is applicable to marine microalgae, with their oils as valuable biodiesel precursors, their carotenoids as high-value antioxidants and animal feed pigments, the residue for further extraction for chemicals and finally for anaerobic digestion, methane production and power generation. The EU Framework Programme 7 calls provide opportunities for international consortia to bring concepts to demonstration stage.
Marine biofuels
Marine biofuels, meanwhile, have become a serious initiative. Estimates put the sector investment in 2008 at over $300m in the US, and this year there is the widely publicised $600m promised by Exxon Mobil for a joint collaboration on algal biofuels with Synthetic Genomics. The USA’s DARPA (Defense Advanced Research Projects Agency) and ARPA-E (Advanced Research Projects Agency – Energy) programmes are putting hundreds of millions of US dollars into this area, providing grants to industry–academic consortia. Iowa State University, for example, is involved in a $4.3m project for metabolic engineering and synthetic biology of algae to improve light capture and oil production efficiencies, while the universities of Arizona and Carolina are working with Diversified Energy on using cyanobacteria for the production of diesel precursor fatty acids.
At least five US companies, including Solazyme, Algenol, Sapphire Energy, Cape Cod Algae Biorefinery and Live Fuels, have already established plants to produce algae for biofuel. Solazyme is supplying material to Honeywell-UOP’s pilot plant for production of 1500 gallons of aviation fuel, and is working with Chevron on a project for the US DoE’s National Renewable Energy Laboratory. Algenol was aiming to exceed its production target of 10,000 gallons of fuel ethanol/acre of bioreactor culture by the end of 2009. Shell and HR BioPetroleum’s joint venture Cellana in Hawaii is currently developing a pilot-scale hybrid system involving bioreactors and managed ponds. However, there are risks that the algal biofuels bubble may have burst in the US, with over-investment in systems too early-stage to deliver and companies feeling the effects of underestimating the costs and scale of the technical challenges.
Macroalgae also have potential for energy production, with the advantage of a lack of lignin that helps offset their high water content. DuPont has a $9m ARPA-funded project on biobutanol from seaweed and ITI Energy in Scotland is funding an integrated programme to establish a realistic anaerobic digestion pathway for combined heat and power from seaweeds. Sustainable, reliable and consistent supply is a challenge, with considerations of ecological impact and consistency of natural harvesting versus the engineering and logistical requirements of near- and off-shore farming. Local power generation is feasible: following the purchase of the marine hydrocolloid company Kelco by ISP Alginates and the takeover of ISP by FMC, there are now ample supplies of Macrocystis giant kelp in southern California that could provide feedstock for an energy plant.
Nano-biotechnology
It isn’t just jet-fuel and methane for combined heat and power that are targets for algal technologies. Researchers at the Ångström Laboratory at the University of Uppsala in Sweden have shown that cellulose nanoscale meshworks obtained from Cladophora, a nuisance macroalgae from the Baltic Sea, can be used as supports for electroconductive polypyrrole polymers, producing a lightweight but power-dense battery – the device is in the early prototype stage but is scalable. The microporous internal environment of incompletely-carbonised shrimp shells has also been shown to improve potassium fluoride catalysis of biodiesel production from methanol and rapeseed oil.
Meanwhile, Israeli company NanoCyte has patented methods of harvesting the stinging systems – nematocysts – from sea anemones and other marine organisms, to provide a novel functional excipient for pharmaceutical products that are applied onto the skin surface. The nematocyst stinging threads penetrate the skin painlessly to produce micropores for transfer of the active pharmaceutical ingredient into the dermal layers. NanoCyte is developing formulations based on this approach with partners interested in acne, pain relief, anti-aging and actinic keratosis.
The challenges ahead
While the opportunities from harnessing marine bioresources are seemingly limitless, obtaining a reliable and sustainable supply of raw materials presents logistic and practical problems. Making sense of biodiversity using high-content screens will still be needed, and some kind of integrated screening and validation system would help, in maintaining innovation flow from originators to marketers. We are really just at the threshold of understanding and using the ‘omics, from metagenomics to synthetic genomics, and applying what we have found in the human genome programme and the Census of Marine Life to the marine biosphere will open new doors.
Meredith Lloyd-Evans is managing director of consultancy firm BioBridge based in Cambridge, UK. This article is based on a presentation at the EFIB meeting in Portugal in 2009.
Further reading
Lloyd-Evans LPM (2004), A Study into the prospects for marine biotechnology development in the UK; see http:// www.berr.gov.uk/whatwedo/sectors/biotech/agribusiness/ biosciencemarine/page10522.html





