Gold nanoparticle-based technologies are showing promise in providing cost effective solutions for environmentally important issues from greener production methods to pollution control and water purification, writes Trevor Keel
Gold is an element that has long fascinated humankind. Its beauty and longevity have made it much sought-after for jewellery and decorative purposes, from the moment it was first discovered until the present day. In more recent years, its malleability, durability and electrical properties have seen the yellow metal become a key material in a number of vital industries including electronics and dentistry.
The recent global economic downturn has seen gold return to prominence in the financial world. The gold price has surged as the market has sought alternative investments, climbing to over $1200/ ounce at the end of 2009. Despite the financial pressures of the last few years, scientific interest in gold is also at an all time high. Key to this has been a range of technological breakthroughs, which are opening up new opportunities for costly materials to be used more sparingly – for example, as thin films or nanoparticles – whilst retaining their effectiveness and durability.
The potential of gold
The field of catalysis is a prime example. For many years, gold was believed to be of limited practical use as a catalyst, despite other precious metals like platinum and silver being widely utilised. It was only in the late 1980s that interest in gold really began to take off. Key studies published by Masatake Haruta at the government research labs in Osaka in Japan and Graham Hutchings working at the University of Witwatersrand in South Africa highlighted the potential of gold for promoting CO oxidation and ethyne hydrochlorination, respectively.1 Since then the understanding and availability of such materials has led to the exponential growth of interest in gold as a catalyst, culminating recently in an entire book on the subject.2

An equally important ‘building block’ chemical is vinyl chloride monomer (VCM). There are two key routes commonly used in the manufacture of VCM – ethene oxidative hydrochlorination and ethyne hydrochlorination. Economic considerations have typically seen the former route dominate, but recent spikes in the cost of oil have seen the coalbased ethyne hydrochlorination process become economically viable once more. This route uses mercuric chloride as a catalyst, which is decidedly ‘non-green’. However, improved gold catalysts and the changing economic landscape mean that mercury could be replaced in this reaction, leading to a more efficient and environmentally sound process for VCM production.3
Gold is also of interest for other commercially important reactions, including the selective oxidation of sugars, the production of methyl glycolate and the water-gas shift reaction. Many major petrochemical and fine chemical manufacturers have been actively producing patents in these areas.4
To cater for this growing interest in commercial gold catalysis, large-scale supplies of gold nanoparticle catalysts are now available from various sources. Global innovation company 3M, for example, has developed a catalyst distributed by UK-based Premier Chemicals, under the trade name NanAucat. The catalyst, which is nanoparticulate gold on a carbon substrate, is highly efficient with low gold loading, making it commercially viable. Mintek also supplies gold-based catalysts on a range of supports under the brand name AuroLITE, all of which are available through Strem Chemicals.
Environmental issues
Outside of these commercial processes, meanwhile, gold catalysts may also have a role to play in helping to combat a number of the world’s pressing environmental challenges. Mercury, for example, is a highly toxic substance found in small ground deposits all over the world. Approximately 150 t of mercury finds its way into the atmosphere every year, a third of it from coal-fired boiler emissions. As mercury has been linked to Alzheimer’s disease and autism, it is anticipated that the US Environmental Protection Agency (EPA) will soon impose stringent limits on mercury emissions from such boilers in the utilities industry.5 Coupled with the fact that the US is relying increasingly on coal to produce electrical power, it is clear that any stringent limits could prove difficult and costly to meet. As such, there is currently a major focus on identifying methods to prevent more effectively the release of toxic forms of mercury into the atmosphere.
Exciting new research is beginning to emerge, which suggests that gold-based catalysts can provide a solution. Studies at the US National Energy Technology Laboratory (NETL), in collaboration with Johnson Matthey, have shown gold nanoparticles to have considerable promise as mercury oxidation catalysts. Full-scale trials are now under way in one US power station. Further research in this field has also been supported by the World Gold Council at Queen’s University Belfast, UK.
Carbon monoxide poisoning is another problem now being effectively tackled by gold catalysts. Often produced as a result of poorly ventilated boilers, CO poisoning hospitalises more than 4000 people in the US every year, with 10% of cases resulting in death. Gold nanoparticles provide a simple solution by catalysing the low temperature oxidation of CO to carbon dioxide (CO2), opening up the possibility of using cost-effective amounts of gold as a commercially viable CO oxidation catalyst in a range of domestic and industrial applications. A number of companies have already developed respirators used in emergency situations for protecting firefighters and miners from CO poisoning, and other applications are likely to appear soon.
Water purification
Yet another area where gold catalysts are showing their metal is in addressing the issue of water quality. Our planet has no shortage of water, but it is unevenly distributed with much of it being undrinkable due to salinity or pollution. In some cases this is as a result of industrial pollution, in others the local geology means that even well water contains significant levels of heavy metals such as arsenic. However, heavy metals aren’t the only problem – other common pollutants include pesticides and halogenated organics. These are all prevalent chemicals in many parts of the world, meaning literally millions of people are at risk of being exposed to contaminated drinking water.
Recent years have seen a sharp rise in the use of noble metal nanoparticles for water purification and contaminant detection. In addition to their potential for oxidising mercury in gas flues, gold nanoparticles have also been shown to be efficient adsorbents for removing significant quantities of mercury from water.
One other recent breakthrough in water treatment technologies has been the development of catalytically active bimetallic gold-palladium nanoparticles for breaking down various chlorinated hydrocarbons, such as trichloroethane (TCE). TCE has a wide range of uses, including as a degreasing agent in the electronic and automotive industries, and in chemical production. However, TCE is known to be one of the most common and poisonous serious human health issues including liver damage, impaired pregnancy and cancer. Palladium catalysts have long been known to break down TCE and related chlorinated compounds in water effectively at room temperature using hydrogen, but high catalyst cost has prevented widespread adoption of the technology.
CHCl = CCl2 + 4 H2 → CH3CH3 + 3HCl
A team in the US has tackled this issue by coating minute quantities of Pd onto gold nanoparticles. Whilst gold is more expensive than palladium, the dramatic increase in the catalytic activity – over two orders of magnitude greater – more than compensates for the slight increase in material cost. Further advantages of using gold in this system have also been uncovered, including an improvement in poisoning resistance of the catalyst itself.6
Looking ahead
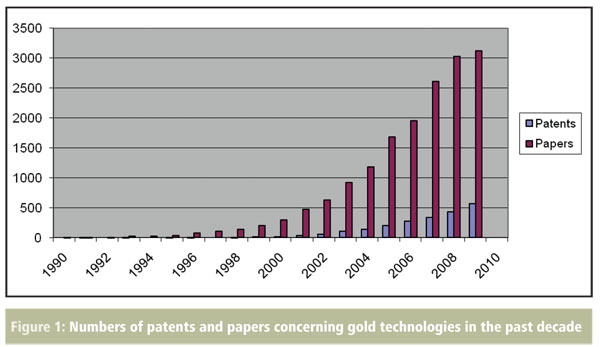
The scientific worth of gold continues to grow unabated. New technological advances, such as the availability of gold in a variety of nanoparticulate forms, have opened up exciting new avenues of research for the metal. One only needs to see the rapid growth of gold nanoparticle-related articles and patents in the recent literature (Figure 1) to get a feel for this expanding field.
In the coming years we expect to see numerous new applications emerge for gold as much of the fundamental research being carried out today begins to bear fruit. More efficient catalysts will be among these, made economically viable by intelligent use of this precious metal.
Trevor Keel is a project manager in the World Gold Council’s Industrial Sector based in London, UK.
References
1. O. Vaughn, Nature Nanotechnology, 2010, 5, 1, 5
2. At worldscibooks.com/chemistry/p450.html
3. G. Hutchings, Chemical Communications, 2008, 10, 1148
4. C. Corti et al, Topics in Catalysis, 2007, 44(1-2), 331
5. http://www.platinummetalsreview.com/dynamic/article/view/52-3-144-154
6. K. Heck et al, Journal of Catalysis, 2009, 267, 97
7. N. de las Heras et al, Energy & Environmental Science, 2009, 2, 206
8. A Kumar et al, Journal of Power Sources, 2010, 195(5), 1401
9. http://www.platinum.matthey.com/media-room/our-viewon-.-.-./the-use-of-gold-in-diesel-autocatalysts/
10. http://www.gold.org/assets/file/pr_archive/pdf/ns_wgc_announcement.pdf





