Following the launch of Coca-Cola’s PlantBottle packaging technology in 2009, efforts to develop a fully bio-based version of polyester or polyethylene terephthalate (PET) – made entirely from plants – have been ramped up with the announcement in June 2012 of a new Plant PET Collaborative (PTC), involving Coca-Cola, Ford Motor Company, H. J. Heinz, Nike and Proctor & Gamble.
PlantBottle technology has been used in more than 10bn bottles and is now available in 24 countries across a variety of the company’s branded products, according to Coca-Cola. It involves using a bio-based version of monoethylene glycol (MEG), currently derived from sugar cane and molasses grown in Brazil and India, respectively, to make PET.
However, MEG accounts for only a third of the raw ingredients used to produce PET, says Coca-Cola’s general manager for PlantBottle packaging, Scott Vitters; the other 70% is purified terephthalic acid (PTA), which has proved more difficult to substitute by a bio-based material. Mixing MEG and PTA together with a catalyst at atmospheric pressure results in an esterification reaction with the loss of water to produce PET (see Scheme).
While Coca-Cola plans to use bio-MEG in the entire group’s virgin PET polymer by 2020, Vitters says that second generation PlantBottles, also made from bioPTA and so made entirely from renewable materials, could be produced commercially in the next couple of years.
’PET plastic delivers half of our beverage volume globally,’ he continues. ‘The environmental benefit of moving to responsibly sourced plant-based ingredient sources for PET is that it can reduce our dependence on non-renewable fossil material and lower our carbon impact while remaining100% recyclable.’
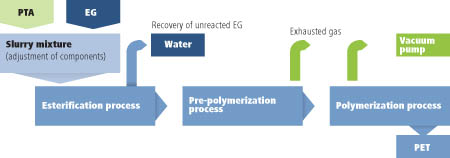
Scheme 1: Production process for PET used in Coca-Cola's Plantbottle
Two of the companies helping Coca-Cola to develop bio-PTA are Virent and Gevo, which both seek to develop biorefinery operations that will deliver the PTA precursor paraxylene (PX) along with other products directly from plant feedstocks. Virent’s technology involves using inorganic catalysts, for example, in fixed bed or continuous reactors, while Gevo’s route entails a yeast-based ‘biocracker’. Both are aiming to develop a variety of drop-in hydrocarbon products that directly replace their petrochemical equivalents.
According to Gevo’s director of chemicals, Bob Bernacki, speaking at the BIO meeting in Boston in June 2012, the company has already produced a 100% bio-paraxylene together with Japanese chemical firm Toray, and used this to make bioPET. ‘We are now working to scale this up to pilot scale and work out the economics,’ he says, adding that paraxylene production results from the C8 side stream of the biocracker, while the company is also exploiting the C12 and C16 fractions as jet fuel.
The company’s major product is isobutanol – a drop-in replacement for petroleum derived isobutanol for all current applications as a solvent and starting point for derivatives such as butyl acetates, acrylates and aldehydes.
More than half of the world’s total PET production – currently some 54m t worth around $100bn – is for synthetic fibres, with bottle production accounting for around a third of global demand. PET is generally referred to as polyester for textile applications, whereas the acronym PET is preferred for packaging.
‘PlantBottle packaging is not just catalysing positive change for bottles, but also across all polyester products – clothing, carpet, car interiors etc – and even broader across all commodity plastics,’ Vitters acknowledges, referring to the launch of the PET collaborative as evidence of the interest in a bio-based PET not just for plastic bottles but also for fibres and other applications.
At Virent, director of business development Kieran Furlong expects that growing demand for polyester for textiles as well as packaging applications will double the world market for paraxylene by 2020 – and triple its value.
Polyester makes up about a fifth of world polymer production and is the third-most-produced polymer, after polyethylene (PE) and polypropylene (PP).





