Alzheimer’s disease (AD) is linked with two pathologies: the accumulation of amyloid-β protein that forms plaques outside neurons, and the accumulation of tau protein that forms tangles inside the neurons. The quest for a disease-modifying Alzheimer’s treatment aimed at these pathologies is littered with high-profile failures.
In 2012, Eli Lilly’s antibody Solanezumab, a monoclonal antibody that binds to β-amyloid protein, failed to improve cognition and function of patients with mild to moderate Alzheimer’s in two Phase 3 clinical trials. This came just weeks after Pfizer and J&J (and partner Elan) dropped development of their anti-amyloid antibody Bapineuzumab after it failed two Phase 3 trials. Most recently, Baxter International’s Gammagard – the only remaining treatment in late-stage development – failed to preserve cognitive performance of patients with mild to moderate Alzheimer’s in Phase 3 clinical trials.
Although drug candidates have not been able to help patients with mild to moderate Alzheimer’s, in some cases there is evidence that they might be effective at an earlier stage of the disease.
Eli Lilly, for example, reports that a secondary analysis of pooled data across both trials showed a significant slowing of cognitive decline in patients with mild AD. The company, with funding from the National Institutes of Health (NIH) in the US, is now testing Solamuzamab in asymptomatic patients.
Marc Wortmann, executive director of Alzheimer’s Disease International, comments: ‘We have seen in some clinical trials, which were carried out recently, that new disease-modifying drugs might only be effective in mild Alzheimer’s disease, which underlines the importance of early diagnosis.’
What is not clear, however, is how to work out which people without symptoms are destined for AD.
Dementia diagnosis
Dementia is diagnosed by measures of both functional and cognitive impairment, but this identifies only those patients with clinical symptoms and in which extensive neurological damage has already been done. Sophisticated computer tools, such as CAMCI (Computer Assessment of Memory and Cognitive Impairment) can detect mild cognitive impairment (MCI) but, again, significant neurological damage may have already occurred by this stage.
Detecting the disease earlier – when the patient has pathological changes in the brain but displays no overt symptoms – is possible via techniques that involve imaging or biomarkers – measurable substances in the body that indicate disease states. Magnetic Resonance Imaging (MRI), for example, can show brain shrinkage, while amyloid and tau accumulation can be seen on PET (positron emission tomography) scans; and levels of biomarkers from spinal taps can indirectly indicate the presence of β-amyloid or tau in the brain. However, these techniques are either unpleasantly invasive or very expensive.
Although such diagnostic tests together provide the opportunity for drug companies to develop effective drug candidates, the criteria for ‘early stage’ disease – exactly how much brain shrinkage, how many plaques and tangles, or the levels of biomarkers, for example – has still not been defined.
‘That is inherently the problem,’ says Tony Butler, analyst at Barclays Capital in New York, US. ‘Early stage implies someone at a certain stage in progression but there’s no standard definition of “early”.’
‘It’s a great challenge in the field,’ agrees Jeffrey Nye, vp and head of neuroscience innovation and partnership strategy at Janssen R&D. ‘We know now from research on various populations that the process begins 10–20 years before the start of dementia/functional impairment. But very few patients are identified or diagnosed [at that early stage].’
The lack of a definition of ‘early stage’ means that patients that could be suitable for clinical trials are not being identified, which helps neither patients nor therapeutic development. ‘We need individuals to be able to identify themselves – that would galvanise public support and enable testing new medicines,’ says Nye.
To help companies, the US Food and Drug Administration (FDA) recently issued draft guidance on the clinical development of drugs for the treatment of early stage disease. In it, however, the FDA points out that the effect of a treatment on biomarker levels has not yet been proven to predict clinical benefit in Alzheimer’s patients.
Different biomarkers are used for different purposes. Some can be used to identify and diagnose people who have changes in brain pathology, while others could be used to monitor changes over time, for example, linking to a clinical outcome in a drug trial. ‘Unfortunately, we’re lacking a drug that provides validation for such a biomarker, in a manner that it can be used as a surrogate for clinical outcome measures – we need a drug that affects both biomarkers and symptoms to claim that connection,’ says Johan Luthman, who leads early development for neuroscience at Merck Sharp & Dohme.
There are two sets of standards for diagnosing pre-dementia AD: one developed by the US National Institute on Aging and the Alzheimer’s Association, and the other, dubbed the ‘Dubois criteria’, developed by a group of international experts. However, the field has not yet settled on one uniform set of criteria that defines ‘early stage’ AD, according to the FDA.
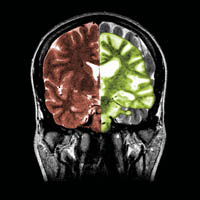
A composite image showing a normal coronal (frontal) cross-sectional MRI image of the brain (in brown) with a superimposed coronal MRI image of a brain with advanced Alzheimer's disease (in green). The diseased brain shows severe generalised atrophy (shrinkage) of brain tissue with an accentuated loss of tissue involving the temporal lobes
Early-stage detection
Recently, researchers in Australia identified a biomarker signature that could lead to an inexpensive blood test to identify people at risk of developing AD and justify more invasive or costly confirmatory tests or early interventions.
As part of the Australian Imaging and Biomarkers Lifestyle Study of Ageing (AIBL), researchers, led by Samantha Burnham and Lance Macaulay, measured the levels of biomarkers in the blood of patients with high levels of amyloid in the neocortex region of the brain, compared with those with low levels. High levels of amyloid in the neocortex can be a predictor of Alzheimer’s disease because there is a strong correlation with shrinkage of the hippocampus – a region of the brain that is important in memory – and degeneration of neurons. They found that the levels of five biomarkers were significantly different between the groups (Molecular Psychiatry, doi:10.1038/mp.2013.40).
Burnham and Macaulay say their team is still looking to improve the ability of the five biomarker panel to screen individuals and are investigating other possible biomarkers. They are validating their results in other populations as well as looking at the logistics of how to format this test for roll out – a process that could take five to 10 years.
‘There are more and more data being generated to show that lifestyle strategies [such as diet, exercise, and intellectual and social stimulation] may slow progression/be protective but to be effective they would require very early intervention,’ say Burnham and Macaulay. ‘The value of the [five-] biomarker panel is that it could provide up to a 20-year window to intervene. This is also really exciting as it enables drug trials targeting the early stages of the disease.’
Early stage drugs in the pipeline
Despite the lack of an official definition of early stage disease, the FDA has proposed accelerated approval for drugs that show promise in treating the earliest symptomatic stages of the disease.
An ideal candidate for these new guidelines, according to Marguerite Prior, a research associate in the Salk Institute’s Cellular Neurobiology Laboratory, US, is J147, a small-molecule compound – a hybrid of curcumin and cyclohexyl-bisphenol A – that is involved in the growth of neurons. J147, which was developed by Prior’s team, can reverse late-stage memory deficits and learning in Alzheimer’s mice, reduce levels of soluble amyloid, and increase proteins that cause neuron growth and are essential for memory, after only three months of oral administration (Mol. Psychiatry, doi: 10.1038/mp.2013.40).
The drug candidate has also been shown to enhance memory in normal animals and prevent memory deficits and Alzheimer’s pathology from developing in early-treated transgenic Alzheimer’s mice (PLoS One, doi: 10.1371/journal.pone.0027865).
In the early development of J147, Prior’s team screened synthetic compounds on living neurons in the lab to find out whether they were effective at protecting brain cells against several pathologies, such as low levels of growth-promoting proteins, associated with old age – the greatest risk factor for Alzheimer’s and other neurodegenerative diseases. In contrast, much of the pharmaceutical industry has focused on the biological pathways involved in the formation of amyloid plaques that characterise Alzheimer’s, thereby modelling disease prevention rather than disease modification. ‘If the therapeutic strategy we used were employed it might prove to be a more rigorous preclinical model to test drugs,’ says Prior.
The next step for J147 is clinical trials, but securing funding is not easy. According to Prior, only 1.6% of NIH funds are spent on Alzheimer’s research, compared with 10% for AIDS, which has a patient population of around only 50,000. In contrast, more than 5m Americans are living with Alzheimer’s disease, the sixth leading cause of death in the country and the only one among the top 10 that cannot be prevented, cured or even slowed, according to the Alzheimer’s Association.
‘We are currently trying to get funding to develop J147, but it has proven exceedingly difficult in part because of the lack of funds at NIH for [Alzheimer’s] drug discovery, and also because with all the failures in clinical trials, [venture capital] companies don’t want to take the risk. Pharma also don’t want to take the risk [so] some are giving up completely on [Alzheimer’s] drug discovery,’ says Prior.
Merck Sharp & Dohme’s Luthman adds: ‘Very little money has come from government so far, but Big Pharma is still committed to funding R&D development for Alzheimer’s disease.’ Merck Sharp & Dohme, for example, currently has a BACE (β-site amyloid precursor protein cleaving enzyme) inhibitor MK-8931 in Phase 2 clinical trials that prevents the formation of β-amyloid peptide. Because BACE inhibitors have not yet been tested in any Alzheimer’s populations, MK-8931 is initially being studied in mild-to-moderate Alzheimer’s patients, but the intention is to test it in patients with early stage disease, according to Luthman.
In March 2013, Merck Sharp & Dohme struck a deal with Luminex to develop a companion diagnostic that will measure two biomarkers in cerebrospinal fluid, to help identify MCI patients who have a higher risk of developing AD. Merck Sharp & Dohme also made a deal in December 2012 with GE Healthcare to use an imaging biomarker to help identify such MCI patients who might benefit from MK-8931.
Roche and Janssen Pharmaceuticals are among those that have BACE inhibitors in development, and Roche (along with AC Immune and Morphosys) has two antibodies in Phase 2 clinical trials that target β-amyloid. Eli Lilly expects its first anti-tau antibody to enter clinical development within the next two years. In April 2013, the company acquired two PET tracers to help it identify tau tangles in Alzheimer’s patients. The diagnostic could help early identification of at-risk patients, and potentially provide a marker for treatment response.
But, as Richard Mohs, leader of early phase neuroscience clinical development at Eli Lilly, points out, in order to make new treatments available for patients: ‘We need to understand how new investigational medicines can be tested in an earlier patient population, and what the regulatory expectations will be for showing their efficacy.’
Meanwhile, there are signs that the issue is starting to be taken seriously by governments. ‘We are very positive about initiatives to increase international collaboration on research like the Joint Programme for Neurodegenerative Diseases (JPND) in Europe and collaboration of international funders started by the Alzheimer’s Association in the USA,’ says Wortmann. The Critical Path Institute in the US is working with the FDA to develop biomarker standards and other tools that could accelerate drug development for AD.
In March 2012, the UK government launched a challenge on dementia that highlighted a need for early diagnosis, along with the need to improve treatments. The Alzheimer’s Research Trust showed that in 2010 dementia cost the UK economy over £23bn – more than cancer and heart disease combined.





