Liver failure due to alcohol abuse, drug overdose and hepatitis is a growing problem. In the US, almost 14,000 patients were waiting for a liver transplant as of February 2018. In 2016, 1220 Americans died waiting for a liver transplant, with the cost of treating cirrhosis – late stage liver scarring – is estimated at nearly $10bn/year.
Transplant surgeon Paulo Fontes at the University of Pittsburgh, US, regularly meets patients who ask what their options are other than a liver transplant. His group has attempted to build a new bioartificial liver, by seeding liver cells onto a liver scaffold; however, others working in this area have so far met with little success. Several patients died after researchers in Sweden tried transplanting a similarly assembled trachea into patients.
‘In 2017, if you have liver failure, we don’t have a backup system,’ says Fontes. ‘But my group has a potential backup system. We are not ready for prime time yet, but we’ve some really good data.’
Now Fontes, working at the Starzl Transplantation Institute, has hit on a different strategy: to grow mini livers in living organisms. The work is in collaboration with Eric Lagasse, a stem cell biologist at the University of Pittsburgh, who showed that the small bean shaped glands called lymph nodes are excellent ‘bioreactors’ for growing different types of cells, including liver cells (Nature Biotechnol., 2012, doi:10.1038/nbt.2379).
Lymph nodes filter damaged cells and foreign particles out of the body’s lymph system, which transports fluids around the body. When someone is ill, T cells from the immune system move to the lymph nodes to be cloned and released back to the bloodstream en masse to take on the microbe causing the illness. The lymph nodes are also where cancer cells commonly migrate to and are replicated before progression to the more serious metastasis disease stage. ‘They are a natural bioreactor,’ and also support the growth of new cells, says Fontes.
For the past five years, Fontes and Legasse have been working with large animal models, infusing hepatocytes into the lymph nodes of pigs. ‘Within two months, it is amazing, but you have mini livers in the lymph nodes,’ he explains. ‘When you compare the mini liver with normal livers, they look very similar.’
|
<4000
|
|
‘Ghost hearts’ are made by removing all of the cells from the heart of a dead human or pig and then repopulating the remaining scaffold with stem cells.
|
The mini livers weigh a few grams and would not offer a complete replacement for failed livers, but rather a supplement of liver tissue in patients with end stage liver disease who are too sick to undergo a transplant. ‘A lot of these patients have significant heart and lung problems, so would not sustain a full transplant,’ says Fontes. ‘The idea is to sustain their life by increasing their liver mass by creating new small ectopic livers within their lymph nodes.’ Liver cells from a donor would be infused into the patient’s lymph nodes, where they would replicate and then be transferred to their liver.
Fontes and Lagasse have started a new company, LyGenesis, and have been in talks with the US Food and Drug Administration (FDA). They plan more work in animals before moving into the clinic.
Meanwhile, Scott Nyberg, a liver transplant surgeon at the Mayo Clinic in Minnesota, US, has ambitious plans for growing entire livers. In around 2010, Nyberg began using pig liver to grow a bioartificial organ called the Mayo Spheroid Reservoir Bioartificial Liver. ‘I made the serendipitous discovery that hepatocytes [liver cells] will self-organise into little liver organoids after you rock them,’ says Nyberg. ‘There is no limit to how many cells you can put in our device, which, from an engineering point of view, is a major advance.’
Nyberg hopes his bioartificial liver will support some patients to allow for natural recovery, or buy enough time for a transplant organ to become available. ‘We recently published a paper showing that this device does improve liver recovery and liver regeneration in pig models of liver failure,’ he explains (J. Hepatology, 2015, 63, 388).
The animal model group with 100% mortality by 72 hours under standard therapy showed 100% recovery after treatment with the bioartificial system. The machine looks similar to a haemodialysis machine, but contains a reservoir of liver cells. He is now trying to raise money to test the device on people to move closer to FDA approval.
Other work by Nyberg’s team, meantime, is aimed at growing up full scale human livers in pigs. ‘Usually people think of taking a pig liver, camouflaging it from the human immune system and transplanting it into humans,’ Nyberg says. He plans to do the reverse: to take human liver cells and transplant them into a pig embryo and use the pig as an incubator. ‘Then you have an organ that can be used either for human transplant or at least use the cells for human treatment,’ he explains.
Already, he has transplanted pig stem cells into mutant pig embryos – a few days old - to create a liver made from two pigs. ‘We’ve truly chimeric pigs born that had a healthy liver composed of cells from a normal donor animal,’ says Nyberg. ‘They would have died without the stem cell transplant and that for me is very exciting. I don’t know when it will be ready for human studies, but that is amazing progress.’
He is now in conversation with the US National Institutes of Health (NIH) concerning how to move the technology forward. One of the major issues will be to address safety, particularly concerns about the possibility that some harmful pig viruses may be transferred to humans. Porcine endogenous retrovirus can infect human cells in test tubes. While viruses are a risk, however, Nyberg believes this is often overblown. ‘My grandfather and uncle were hog farmers. Butchers and farmers have been around pigs for hundreds of years without disease transmission,’ he notes. ‘I am a transplant surgeon. The livers I am transplanting into humans from other humans are [sometimes] much riskier than pigs from an FDA-approved [disease free] facility, which are carefully screened beforehand.’
Artificial hearts
Compared with artificial livers, artificial heart technology is much further along the road to the clinic. To date, around 2000 artificial hearts have already been implanted in patients, with demand driven similarly by an acute shortage of donors. Fewer than 4000 donor hearts become available each year, while around 100,000 patients await the operation at any one time, according to the International Centre for Artificial Organs and Transplantation.
One problem, however, is that only one standalone artificial heart has been approved for use in the US and EU. SynCardia was developed in the 1980s, but remains a bridge to a transplant rather than a replacement. It can cause many adverse events, says Nicholas Cohrs at ETH Zurich in Switzerland. Risks include wearing out or failure of the electrical motor, infection, and the need to take blood thinners to prevent clotting. Stroke and bleeding are also possible complications with the artificial heart. Around two-thirds of patients are supported by these artificial hearts for less than a year.
Cohrs is among a coterie of scientists hoping to develop artificial organs that mimic nature more closely. ‘Syncardia is made of rigid materials unlike the human heart,’ says Cohrs. In his lab in Zurich in Switzerland, an experimental soft artificial heart chugs away in the background. Unlike most heart devices, this one is made from silicone and inflates – then deflates to pump blood (Artificial Organs, 2017, doi:10.1111/aor.12956).
‘We wanted an artificial heart very similar to the natural human heart,’ explains Cohrs. ‘Our hypothesis is that when you mimic the human heart in function and form you will have fewer side effects.’
Eighty percent of patients won’t in fact need a total artificial heart, and will recover function sufficiently with a so-called heart assist device. These devices don’t provide a replacement heart, but typically use propeller systems to drive blood from the left ventricle and around the body. To date, about 100,000 ventricle assist devices, or heart pumps, have been surgically inserted. The two most common assist devices are Heart Mate II and Heart Ware, which propels the blood with a rotor resembling a witch’s hat.
‘The downside of these devices is that they still have complications, the most common being infection, stroke, bleeding and heart rhythm problems,’ says cardiac surgeon Robert Kormos, at the University of Pittsburgh.
|
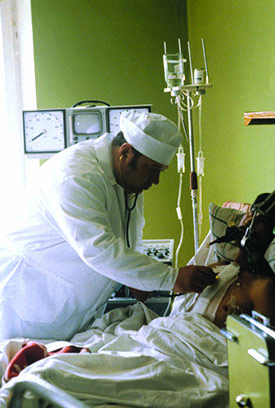
Russian-Soviet transplant surgeon Valery Ivanovich Shumakov with a patient on a ward. Shumakov specialised in researching blood flow and the heart. He performed the first heart transplant in Russia, as well as the first liver and thyroid transplants. Image: Sputnik/Science Photo Library
|
Another problem is that assistive pumps do not generate a pulsatile flow, he continues – the patient has no pulse. ‘Nature has given us a pulse for a reason. A lot of our endocrine organs and vascular system response to a pulse in subtle ways. It is probably why those with these devices can suffer gastrointestinal bleeding. It also puts constant pressure on the aortic valve, which then needs to be replaced in 20 to 30% of patients after two years.’
Several companies are currently developing new pulsatile systems, however, such as TORVAD from Windmill Cardiovascular, which senses cardiac rhythm. ‘In the next two to three years, we are going to see a resurgence of pulsatile systems,’ says Kormos.
And there is progress too for total artificial heart technology. In November 2017, for example, French firm CARMAT announced the first implantation of its bioprosthetic artificial heart in the Czech Republic, as part of a trial involving about 20 patients suffering from heart failure. The device weighs three times that of an average human heart, is made of soft biomaterials and works off a lithium battery.
Nevertheless, says Mario Deng, professor of medicine at University of California, Los Angeles: ‘Total artificial heart is still a halfway technology.’ The hearts are placed as a stopgap in patients awaiting a natural heart transplant. ‘We still feel that these total artificial heart transplants can only be recommended in very circumscribed cases,’ says Deng; patients should be at risk of dying in the next six to 12 months, but still have good recovery potential.
Deng ran the heart transplant programme at UCLA between 2011 and 2016, which has fitted eight 70cc SynCardia hearts and taken part in a clinical study of a 6.2kg Freedom device – a 6kg backpack-sized device from SynCardia powered by lithium ion batteries. Neither ventricle assist devices nor the total artificial hearts can be powered internally, he notes, but instead must be connected to an external power source, which can also lead to infection.
Back at ETH Zurich, meanwhile, Cohrs and his colleagues aim to print their artificial hearts so that they fit precisely into an individual patient. This is not yet close to clinic, but promises a tailored heart. ‘We take a CT scan of a patient, put it in a computer file and design an artificial heart around it, so it closely resembles the patient heart,’ says Cohrs. ‘We use these 3D printers and print a mould in ABS [acrylonitrile butadiene styrene], which is the plastic Lego is made of, fill it with silicone and then dissolve the mould with acetone to leave behind the silicone heart.’
The plastic heart deflates and inflates with pressurised air. The first generation device, made from silicone, has two chambers but survived for only 2000 beats. ‘This is only half an hour, so there is a lot of improvement needed,’ adds Cohrs. A new prototype made from a more resistant [so far, undisclosed] polymer has managed more than a million beats, which is the equivalent of 10 days for a human heart. The goal is to develop a four-chamber heart that beats for 10 years, so a lot more work is still needed.
Another strategy, pioneered by Doris Taylor at the Texas Heart Institute in Texas, US, is to take an organ from a dead human or pig and remove all the cells from it, leaving behind a scaffold of collagen and other structural proteins. Then, repopulate the ‘ghost heart’ with stem cells.
‘There is a lot of optimism that re-cellularising hearts has the potential to create a designer heart that can be put into a patient with heart disease,’ says Kormos, ‘but I don’t see that happening quickly.’ The challenges are enormous. ‘It is not about just putting in one cell type. You need all sorts of types and you need them to grow in the correct proportions,’ explains Georgina Ellison, heart physiologist at King’s College London, UK. ‘A rat heart is tiny. You need a lot more cells for a human heart.’
‘It would be great if we could build organs,’ she continues, ‘The holy grail. But it will take a lot more time, funding and manpower.’
Others, meanwhile, are trying to combine synthetic systems with cells on a smaller scale than the ghost hearts. Researchers in Switzerland are designing a heart assist device with a membrane made of polymer and endothelial cells. ‘We are creating surface conditions so that the pump can be covered by endothelial cells from the patient themselves,’ says Mazza Eduardo at ETH Zurich. His team used vulcanised elastomers to coat a pump imprinted with a special surface topography to allow the cells stick to it and tested this in sheep.
So how long before we see an artificial heart being transplanted into human patients? ‘I’m going to stick my head out a little bit,’ Kormos says, ‘and say it is going to be at least five to six years before that comes to fruition.’
|
14,000
|
|
Mini livers weighing a few grams could supplement liver tissue in patients with end stage liver disease who are too sick to undergo a transplant.
|
|
Pigs are being investigated as potential incubators for growing full size human livers for transplantation back into people.
|





