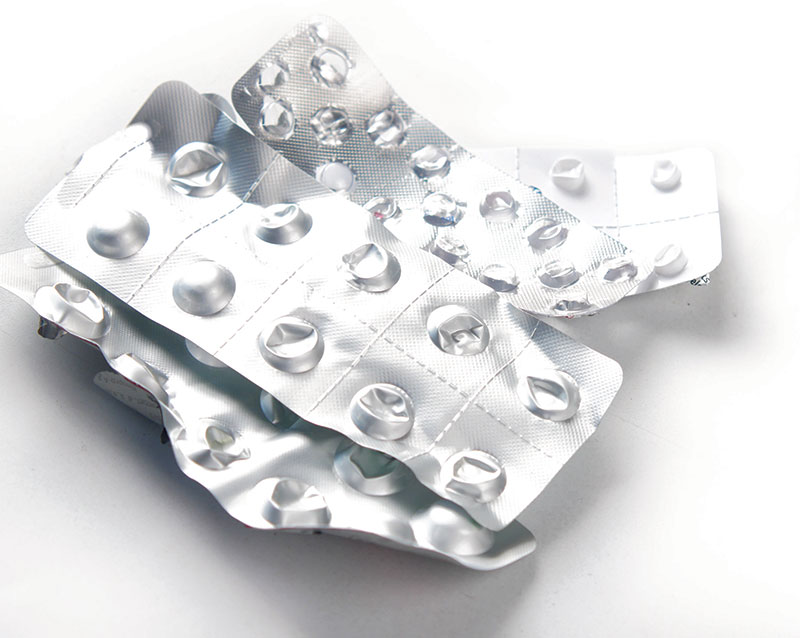
Pharmaceutical packaging, especially blister packs, are a complex mix of materials, which makes recycling difficult. Katrina Megget explores how to make it more eco-friendly
Plastic is the mainstay of most pharmaceutical drug packaging, whether it’s blister packs, bottles, pre-filled syringes or inhalers. Some 100,000t of plastic are produced globally for medicine packaging every year – although just a drop in the ocean compared with the 36m t produced across all industries. Blister packs are perhaps the most ubiquitous pharma packaging – 85% of solid unit drug doses are packed in this form in Europe, and globally the blister packaging market is expected to be worth $24.26bn by 2024, according to Mordor Intelligence.
‘While the recyclability of food and drink packaging has been in the spotlight and a focus for some time, pharma packaging is a sector that is often overlooked in terms of packaging recyclability, perhaps due to the product being a necessity rather than a choice,’ says Katherine Fleet, Head of sustainability and circularity at UK charity RECOUP (RECycling Of Used Plastics), which has recently set up a working group to identify challenges and opportunities in the sector. ‘Consumer awareness of sustainability issues has increased over recent years and there will be a need to meet consumer expectations to remain in the market.’
Indeed, there is already pressure. The UK Plastics Pact, for instance, of which RECOUP is a signatory, wants to eliminate problematic or unnecessary single-use packaging and make 100% of plastics packaging reusable, recyclable or compostable by 2025, blister packs included. ‘We need to move away from the linear model of take, make and dispose, to a circular economy, where resources are valued and never enter the natural environment,’ says Helen Bird, Strategic Engagement Manager for plastics at British charity WRAP, which leads the UK Plastics Pact.
But there is a hitch – pharmaceutical packaging is complex and recycling is not straightforward. For starters, there are four types of pharmaceutical packaging: primary packaging, such as blister packs or vials, which is in direct contact with the drug; secondary packaging, predominantly boxes made from paper or cardboard, which protects the primary packaging; tertiary packaging, which protects the secondary packaging during transport; and ancillary packaging, such as seals or shrink wrap that secures the tertiary packaging. While most of the secondary and tertiary packing materials are made from board that can be recycled, it’s the primary packaging that is most problematic as it is more difficult to recycle and can’t easily be replaced.
Blister packs are a case in point. Introduced in the 1960s, they revolutionised unit dose drug administration while preserving product integrity, but they are a complex mix of different materials, often featuring an aluminium foil pierceable top and rigid plastic or aluminium base material that separates the doses, which are joined together by a bonding agent. This design makes separating the layers for recycling a challenge and most blister packs, as a result, are not accepted by council recycling systems, instead ending up in landfill or incinerated.
PVC alternatives
Meanwhile, the plastic polymer layer adds another challenge. This is typically polyvinyl chloride (PVC) and occasionally polyvinylidene chloride (PVDC). Neither are widely recycled and can be a contaminant to other plastics recycling. That said, PVC has been the primary packaging of choice within the pharma industry, Fleet explains. ‘While PVC is identified as a problematic plastic for recycling it can often be the right polymer in the pharmaceutical setting due to its unrivalled performance characteristics [being versatile, durable, long-lasting and temperature stable], its cost-efficiency and being compatible with virtually all pharmaceutical products. It also has excellent water and chemical resistance, helping to keep solutions sterile.’
Because pharma’s first priority is patient safety, the onus is on the industry to meet specific packaging regulations that focus on protecting the medicine from degradation above any environmental or sustainability concerns. As such, materials can’t simply be switched. Not only would this require lengthy approval but PVC alternatives and material containing recycled plastics don’t meet the stringent safety requirements – resulting in a reliance on PVC, explains Steve Hoare, Quality, Regulatory Science and Safety Policy Director at the Association of the British Pharmaceutical Industry (ABPI).
It’s for this reason that primary pharmaceutical packaging is exempt from the UK government’s plan to implement a tax of £200/t on all plastic packaging components that don’t contain at least 30% recycled material. ‘Being unable to produce pharma grade recycled plastics is a technological challenge, meaning there’s only so much that companies can do to reduce the amount of new plastic they use,’ says Hoare.
There are other challenges facing pharma packaging. In some cases, labels, particularly on over-the-counter bottles, are too big and are made of different materials, making recycling difficult, while blister packs are often disposed of inside their cardboard boxes rendering the cardboard box unrecyclable. In any case, most pharmaceutical packaging contains no pack disposal instructions anyway because it’s not deemed clinically relevant information. Meanwhile, the small size of some primary packaging would have it rejected at sorting facilities even if it was collected for recycling, but even then recycling pharmaceutical packaging isn’t deemed cost effective. In addition, there is a separate safety consideration, says Ana Nicholls, Managing Editor of the Industry Briefing at The Economist Intelligence Unit. ‘The contents, and even the packaging, can often be toxic [due to drug contamination], making it hard to recycle or even incinerate safely.’
While PVC is identified as a problematic plastic for recycling, it can often be the right polymer in the pharmaceutical setting due to its unrivalled performance characteristics, its cost-efficiency and being compatible with virtually all pharmaceutical products.
Katherine Fleet Head of sustainability and circularity at UK charity RECOUP
Green shoots
Despite all this, steps are being made to go green. In August 2021, UK-based contract packing firm Central Pharma Contract Packaging won a share of almost £2m from UK Research and Innovation to develop sustainable packaging solutions, while technology such as 3D printing can minimise waste. There are also studies considering polyethylene (PE), polyethylene terephthalate (PET) or polypropylene (PP), as recyclable alternatives to PVC.
In April 2021, global packaging company Amcor introduced its new blister system AmSky that eliminates PVC and improves recyclability, by using a PE thermoform blister and lidding film. ‘The intrinsic physiochemical properties of the PE heavy structure and Amcor’s proprietary manufacturing process delivers similar barrier levels equivalent to PVC/PVDC and similar mechanical properties, allowing AmSky to run in standard thermoform pharmaceutical machinery,’ says John Forsyth, Senior Product Manager pharma, EMEA, at Amcor. ‘In addition, the PE core structure for both the top and the thermoformed bottom allows for it to be recycled in the traditional PE rigid or flexible streams available in many European countries.’ There is also the benefit of a 70% reduction in the packaging’s carbon footprint, compared with PVC. Forsyth says the firm is currently trialling the packaging with a number of pharma companies and aims to have the packaging available on the market by the second half of 2022.
Meanwhile, Danish supplement firm Natupharma has developed a fully biodegradable plastic bottle packaging solution, with potential for pharma applications. The bioplastic is a carbon neutral, biobased polyethylene obtained from sugarcane-based ethanol that contains an additive to promote plastic degradation in under 10 years, says Martin Greaves, Sales Director at the UK division of Natupharma. Importantly, the additive doesn’t affect physical properties and is not an oxodegradation additive, he adds.
Oxodegradation additives have come in for recent criticism for their ability to accelerate the production of problematic microplastics. Instead, the Natupharma additive enables microbes to secrete acids that degrade the plastic into CH4, CO2, biomass and water. ‘As its robust properties are identical to a regular polyethylene, it can be recycled the same way, as it’s fully compatible with the recycling stream of the petrochemical polyethylene,’ Greaves says. The bioplastic has been tested and is EU compliant for food contact materials and nutraceuticals but question marks remain for pharmaceuticals and the more stringent testing required, he says. ‘We have had a very positive response from pharma companies, but the challenge is always the validation and this can take time.’
£200
Primary pharmaceutical packaging is exempt from the UK government’s plan to implement a tax of £200 per metric tonne on all plastic packaging components that don’t contain at least 30% recycled material.
100%
The UK Plastics Pact wants to eliminate problematic or unnecessary single-use packaging and make 100% of plastics packaging reusable, recyclable or compostable by 2025, blister packs included.
10 yrs
Danish supplement firm Natupharma has developed a fully biodegradable packaging solution – a carbon neutral bioplastic obtained from sugarcane-based ethanol. It contains an additive to promote plastic degradation in under 10 years.
3760
Novartis is aiming to eliminate PVC from all secondary and tertiary packaging by 2025. Its Brazilian unit turns unused blister packs into around 3760 doors annually.
Recycling refined
With new plastic options constrained by safety regulations, the alternative is to refine the recycling process. In September 2020, the UK division of US-headquartered recycling firm TerraCycle joined forces with pharma company Sanofi’s consumer healthcare business in a bid to do just that. With funding from Sanofi, the Medicine Packet Recycling Programme was able to address not just the recycling challenge but the economic challenge of recycling blister packs.
Consumers drop any empty blister packs for free at participating pharmacies, which are then returned to TerraCycle. The firm removes any contamination and washes the material, then shreds it, reducing it to flakes or powder. The aluminium content is separated out from the plastic by density separation before being sent for smelting and recycled as nuts and bolts. Meanwhile, the plastics go through a melting process where they become a usable raw material pellet, which can be extrusion moulded into new products such as window frames or outdoor furniture.
‘The programme saw an encouragingly high uptake,’ says TerraCycle spokesperson Stephen Clarke. ‘However, the resources set up at the beginning of the project could no longer accommodate the large quantities of blister packets we were receiving.’ Several months in, pharmacy chain Superdrug took over operational control of the programme and, along with other pharma companies, the scheme continues to be offered.
Sanofi hopes more pharma companies will come on board as the environmental benefits are recognised. ‘We know we have a role to play to look after our planet and provide healthcare in the most respectful, considerate and sustainable way possible,’ says Harsh GK, General Manager for Sanofi’s consumer healthcare business in the UK. ‘Initiating the pilot with TerraCycle a year ago, diverting hard-to-recycle empty medicine blisters from landfill, was an obvious opportunity for my team to help manage post-use waste generated by our industry.’
Meanwhile, Germany’s Merck KGaA has launched a programme as part of its sustainable packaging strategy to tackle the packaging protecting glass reagent bottles. The company has replaced unrecyclable expanded polystyrene known as Styropor with moulded parts made of cellulose and recycled paper pulp, which can be recycled alongside other paper materials. Novartis is also aiming to eliminate PVC from all secondary and tertiary packaging by 2025 and is working on concepts for how it might replace PVC in primary packaging. Already its Brazilian unit turns unused blister packs into around 3760 doors annually.
‘The pharmaceutical industry is committed to environmental and sustainability targets and playing the biggest possible role in the global drive to net-zero,’ says Hoare. ‘Companies are committed to finding solutions… We are seeing companies do some really interesting things to reduce packaging waste… [But] there’s no one-size-fits-all solution like there might be for other sectors because medicines, medical devices and vaccines vary between companies and have to meet strict regulatory standards of safety.’
Fleet agrees pharma’s attitude is changing and there is more appetite within the industry for sustainability, recognising the benefits for both environment and business. She believes the way forward is two-fold, combining both packaging design and recycling technology. ‘Recyclability by design is key to recycle more plastic from this sector. If designers can give thought when designing pharma packaging and limit where possible the use of colours, multi-materials and label size this would see an increase of packaging from this sector being recycled. There is also a role to play for investigation of alternative materials that may increase the likelihood of capturing material for recycling.’ She also points to advanced recycling technology, such as chemical recycling, which can take more contaminated waste streams. ‘This could prove a potential opportunity for pharma packaging that cannot be recycled via traditional routes.’
However, Fleet also believes recycling regulation for the pharma industry is unlikely, particularly for primary packaging, because of the patient safety considerations. While Nicholls notes regulation could boost innovation, it would need to be flexible for pharma and ‘we are not yet at the stage where firm concrete requirements can be set to make packaging recyclable; it’s more a question of setting targets and then forcing companies to find ways to meet them’. Even Bird acknowledges that for pharma, the 2025 target of 100% elimination is a stretch and that, more likely, it will take longer than four years. But it should be a long-term goal, she says. ‘The move away from PVC is the answer. WRAP believes alternatives are possible.’
This could take years but, in the meantime, there are short-term wins, according to Gregor Anderson, Managing Director at UK-based pharma packaging consultancy Pharmacentric Solutions. Opportunities, for instance, abound within the healthcare system for collection points similar to the TerraCycle scheme. ‘It’s simple really,’ he says. ‘Get a lean, aligned and cohesive system in place with all stakeholders – the NHS, patient groups, suppliers, and wider pharma industry working together on this. Then publicise it to all patients with a simple message. Make patients part of the solution.’