BY SIMON FROST | 8 NOVEMBER 2023
Could today's flue gases be tomorrow's fuel?
Researchers at MIT and Harvard University, US, have developed a new process that can convert carbon dioxide from flue gases or air into formate – a material that can be used like hydrogen or methanol to power a specialised fuel cell and generate electricity (Cell Reports Physical Science, doi: 10.1016/j.xcrp.2023.101662).
Potassium or sodium formate is already produced at industrial scales and considered benign, according to national safety standards. It is commonly used as a de-icer for roads and pavements, and is non-toxic, non-flammable, and easy to store and transport. The researchers also claim it can remain stable in ‘ordinary steel tanks’ to be used months, or even years, after its production.
The MIT and Harvard researchers – supported by the US Department of Energy Office of Science – have demonstrated, at laboratory scale, a process from capture to electrochemical conversion of gases to a solid formate powder, which can then be used in a fuel cell.
MIT professor Ju Li explains that other approaches to converting carbon dioxide into fuel usually involve a two-stage process. First, the gas is chemically captured and turned into a solid form as calcium carbonate; and that material is then heated to drive off the carbon dioxide and convert it to a fuel feedstock such as carbon monoxide.
That second step is where efficiency is lost – typically converting less than 20% of the gaseous carbon dioxide into the desired product. By contrast, the researchers report a conversion of well over 90%, eliminating the need for the heating step by first converting the carbon dioxide into an intermediate form – liquid metal bicarbonate.
They then electrochemically convert this into liquid potassium or sodium formate in an electrolyser, using a low-carbon electricity source. The highly concentrated liquid potassium or sodium formate solution produced can then be dried – for example, by solar evaporation – to produce a solid powder that the team reports is highly stable.
Several factors account for the greatly improved efficiency of the process. Previous attempts at such a system have suffered from a buildup of chemical byproducts that alter the pH, causing the system to steadily lose efficiency over time.
This required careful design of the membrane materials and their configuration. ‘Traditionally, it is difficult to achieve long-term, stable, continuous conversion of the feedstocks,’ said co-author, MIT doctorial student Zhen Zhang. ‘The key to our system is to achieve a pH balance for steady-state conversion.’
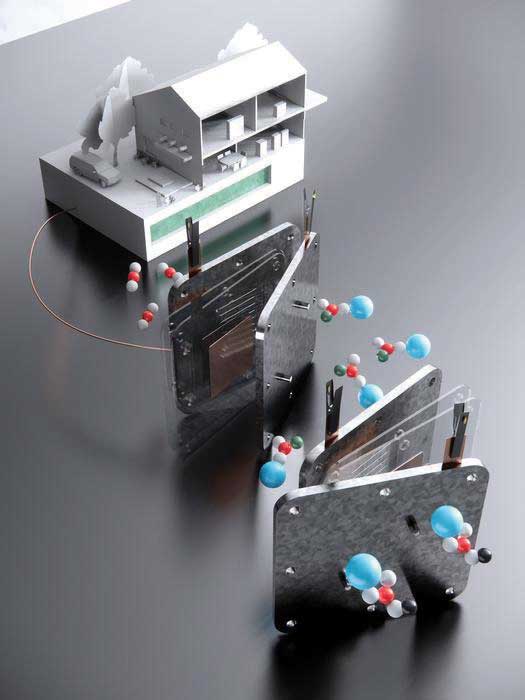
Schematic showing the formate process. The top left shows a household powered by the direct formate fuel cell, with formate fuel stored in the underground tank. In the middle, the fuel cell that harnesses formate to supply electricity is shown. On the lower right is the electrolyser that converts bicarbonate into formate. Image: Shuhan Miao, Harvard Graduate School of Design
To achieve that, the researchers carried out thermodynamic modelling to design the new process to maintain the pH balance over time, allowing it to continue operating efficiently over long periods. In their tests, the system ran for over 200 hours with no significant decrease in output. The whole process can run at ambient temperatures and relatively low pressures (about five times atmospheric pressure). Another issue was that of unwanted side reactions, which the team prevented by introducing an extra ‘buffer’ layer of bicarbonate-enriched fibreglass wool that blocked them.
The team created a fuel cell specifically optimised to use formate fuel to produce electricity. The stored formate particles are dissolved in water and pumped into the fuel cell as needed. Although the solid fuel is much heavier than pure hydrogen, Li notes that when the weight and volume of the high-pressure gas tanks required to store hydrogen is considered, the end result is an electricity output near parity for a given storage volume.
The researchers expect the process to be scalable, and believe it could provide emissions-free heat and power to individual homes, and potentially even industrial or grid-scale applications.
Initial household applications might involve a fridge-sized electrolyser unit that captures and converts the carbon dioxide into formate, which could be stored in an underground or rooftop tank. Then, when needed, the powdered solid would be mixed with water and fed into a fuel cell to provide power and heat. ‘This is for community or household demonstrations,’ Zhang says, ‘but we believe that also in the future it may be good for factories or the grid.’
David Bott, SCI’s Director of Innovation notes: ‘An electrochemical fixing process for low-concentration CO2 that produces a fuel cell input is certainly interesting, but it’s still at the lab-scale and there will be a lot of work to do to catch up with other systems.’





