FEATURE BY MICHAEL GROSS | 20 DECEMBER 2023
From bacteria to mice, worms and fish, other species are sparking fresh insights into the science of ageing, Michael Gross reports
With the global rise in life expectancy, the world is now hosting more centenarians than ever. There are many theories based on anecdotal observations of the few hotspots where people appear to live longer, such as Sardinia and parts of Japan. Now that centenarians are numerous enough to make statistically relevant sample sizes, these kinds of studies will hopefully be more helpful.
Joachim Johansen from Harvard University, US, and colleagues, for example, studied the gut microbiomes of 176 healthy centenarians from Japan and 19 from Sardinia in an effort to find clues to longevity[1].

Life expectancy has increased significantly over the past half-Century.
They discovered that both the bacteria and the viruses (bacteriophages) that attack them were comparatively more diverse than in groups of over 18- and over 60-year-olds. They also discovered new genera of phages in the centenarians, including some associated with Clostridia bacteria. Assisted by phage genes, the bacteria were able to support a wide range of metabolic reactions – some of which may be linked to resistance to pathogens and disease.
‘Intestinal bacteria are a natural part of the human body and of our natural environment,’ said study co-author Simon Rasmussen. ‘And the crazy thing is that we can actually change the composition of intestinal bacteria. If we know why viruses and intestinal bacteria are a good match, it will be a lot easier for us to change something that actually affects our health.’
Anticipating old age, most people will be worried about their minds rather than their guts, with dementia becoming a more widespread problem and some milder forms of mental decline being considered normal. In this context, mental activity is often cited as a way to keep the mind agile. In a recent contribution to this field, Lei Zhang from the Chinese Academy of Sciences at Beijing and colleagues studied the performance in a specific task – audiovisual syllable recognition in the presence of noise – among older people with lifelong musical training, as compared with older non-musicians and younger non-musicians[2].

A recent study found that those with lifelong musical experience were better equiped for audiovisual speech-in-noise perception.
The authors conclude from their observations that ‘lifelong musical experience mitigates age-related declines in audiovisual speech-in-noise perception by preserving youth-like speech representation patterns in bilateral sensorimotor regions,’ while also enhancing specific compensatory mechanisms.
While some environmental factors and lifestyle choices can keep us healthier into old age, other factors carry the risk of accelerating decay. Stress is widely blamed for premature ageing and shorter life expectancy. Recent research suggests, however, that bioindicators of ageing that have gone up in acute stress situations can bounce back once the stress is relieved.
Jesse Poganik from Harvard Medical School, US, and colleagues studied blood samples of patients undergoing stressful medical procedures such as hip operations or experiences, such as being hospitalised with Covid-19[3]. They analysed so called ‘biological clocks’ based on DNA methylation, used as bioindicators for age-related morbidity and mortality. The authors found these clocks went forward, indicating that stressful experiences appeared to make patients age more quickly. In some but not all situations, the biomarkers returned to their previous trajectory after the patients had recovered from the medical situation that caused the stress.
Model studies
Human studies into ageing are necessarily observational. As it would be neither ethical nor manageable to manipulate human patients experimentally and then determine their lifespan after that, experimental research relies heavily on models from across the tree of life. But which model is best?
The worm Caenorhabditis elegans has been a standard model of development and genetic research since the 1970s. It has also been used in ageing research, based on the discovery that suppressing specific genes can extend its lifespan by a factor of ten. However, a recent discovery has cast doubt on its suitability for this purpose.
Carina Kern from University College London, UK, and colleagues suggest that C. elegans belongs to the very small group of animal species that only reproduce once and then sacrifice themselves or die from exhaustion after investing all their resources in the wellbeing of their offspring, so-called semelparous species. Other examples include Pacific salmon and octopuses.40 years ago “fountain of youth” genes were discovered in C. elegans. When suppressed, the animals live even 10x longer. Scientists have tried & failed to replicate the same effects in humans. But why? Check out our paper in @NatureComms that finally answers the question #ageing https://t.co/stX2NIGj7A
— Carina Kern (@CarinaCarlaKern) July 24, 2023
In the case of C. elegans, the London group had previously described a self-destructive process called yolk venting, in which the worm secretes a milk-like fluid consumed by its offspring to support growth. Now Kern and colleagues show that this mechanism is specific to hermaphroditic C. elegans, which are also short lived[4]. The previous observation of life extension thus simply relates to suppressing the reproductive mechanisms that normally kills the reproducing worm early.
Humans, on the other hand, reproduce multiple times, as we are iteroparous like most mammals. We can also survive the life stage of reproduction by decades. All is not lost, however, as Carina Kern and David Gems explain in a recent review[5]. Strategies like the post-reproductive self-destruction of C. elegans may well be evolutionarily related to the more gradual decay in mammalian ageing. So, comparing these two strikingly different approaches to lifespan may still yield useful information.
An emerging model system for ageing is the African turquoise killifish, Nothobranchius furzeri. Adapted to the seasonal rain in its habitat, the species has the shortest lifespan of any vertebrate successfully bred in captivity. The killifish hatch when seasonal rain pools form, mature within two weeks, and then reproduce until the pools dry out again. Remarkably, in their short lives, they go through many of the signs of ageing that humans only experience after many decades of life, including telomere shortening and neoplasms of the liver and the gonads. Avnika Ruparelia from Monash University, Australia, and colleagues have now shown that killifish is also a suitable model to study age-related decline in muscle mass and strength (sarcopenia)[6].
While most aspects of muscle performance declined as expected, the authors also discovered a group of features that appeared to recover in extremely old killifish. This matched the observation also made in humans and other species that individuals far beyond the normal life expectancy have a lower risk of dying than one would expect from extrapolation.
To investigate the mechanisms underlying this reduced mortality, the authors studied the metabolism of the skeletal muscles and proposed a model whereby the animals deplete their triglyceride reserves, mimicking a low-calorie situation. This leads to the activation of the gene sirt1, which is known to trigger multiple changes such as the induction of antioxidant genes, and thereby reduce mortality and thus increase lifespan. ‘The idea that muscle ageing may be reversible, and potentially treatable by drugs that can manipulate a cell’s metabolism, is an exciting prospect especially given the social, economic and healthcare costs associated with the ever-growing aged population around the world,’ Ruparelia said.
Time turner
Taurine supplementation increases healthy life span, according to new research. Credit: Columbia University Irving Medical Center
Using a more conventional model system, the mouse, researchers recently came across a chemical that may just slow down ageing in humans too. Parminder Singh and colleagues from the National Institute of Immunology, New Delhi, India, found that the amino acid taurine, a common ingredient in energy drinks, extended the lifespan of mice by 10 to 12% and boosted the health of macaques[7].
Based on the observation that taurine concentrations in the blood decline with age in mice, monkeys and humans, the researchers fed mice with taurine supplements from the middle to the end of their lives. Beyond the significant increase in lifespan, compared with a placebo control, they also found an increase in healthy lifespan, with improved functioning of bone, muscle, pancreas, brain, fat, gut, and immune system compared with the placebo group.
Investigating the mechanisms of this effect, the researchers found that taurine deficiency is linked to several common health problems of ageing including abdominal obesity, hypertension, inflammation, and prevalence of Type 2 diabetes. As exercise is known to stimulate the body’s own production of taurine, they speculate the compound may be an important reason behind the positive effects of keeping active.
As the amounts used in the study are much larger than the levels included in energy drinks, one cannot assume a comparable treatment would be safe for humans. The authors therefore called for clinical trials in their conclusion: ‘To test whether taurine deficiency is a driver of aging in humans as well, long-term, well-controlled taurine supplementation trials that measure health span and lifespan as outcomes are required.’
While taurine and similar metabolic interventions could end up offering safe routes to healthier and longer lives, others may be riskier, especially those involving stem cells or genetic manipulation. Zhen Zhou from the University of California at San Diego, US, and colleagues manipulated yeast cells, which normally have two distinct pathways towards cell death[8]. By engineering in an oscillating switch, essentially making the cells go back and forth between these two paths, the researchers managed to extend their lifespan by 82%.
Stem cell research has resulted in the development of genetic methods to turn back the clock of differentiated cells in the production of induced stem cells. While this could in principle be called a rejuvenation, it also carries the risk of uncontrolled growth leading to tumour formation. In an effort to bypass this risk, Jae-Hyun Yang from Harvard Medical School, US and colleagues have developed a chemical rather than a genetic protocol to turn back the cellular clock on human skin cells held in culture[9].
‘Until recently, the best we could do was slow ageing. New discoveries suggest we can now reverse it,’ said lead investigator David A. Sinclair.
However, other researchers are more cautious and hope for a better understanding of the big picture. ‘Ageing theory has also so far been obsessed with single causal explanations of ageing, partially stemming from the discovery of these fountain of youth genes,’ said Carina Kern of the C. elegans study. ‘Instead, what we need is an approach that is more holistic and accounts for the multiple biological mechanisms at play – a molecular pathway map, if you will.’
All roads lead to PF4
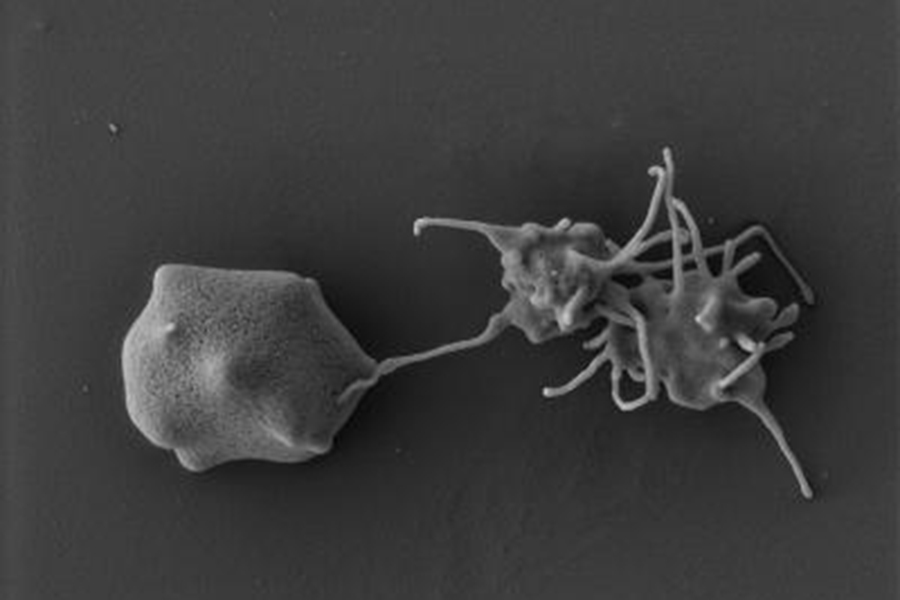
Electron microscopy image of an activated platelet binding to a malaria-infected red blood cell. Image: Andreas Greth, Macquarie University
Three recent model studies on ageing in mice surprisingly converged on the same factor apparently rejuvenating the rodent brain, namely the protein platelet factor 4 (PF4). Adam Schroer and colleagues at the University of California at San Francisco, US, studied the effect that blood infusions from younger mice can rejuvenate older ones[10]. Narrowing down to the causative principle, the researchers first identified platelets – a type of blood cells involved in wound repair – and then the protein PF4.
Injection of this protein alone can revert mice of the age equivalent to 70-year-old humans to a brain health corresponding to half that age. The researcher also identified the receptor involved in mediating this effect, which could become a target for drugs to be developed against age-related cognitive problems.
Simultaneously, a separate UCSF team led by Dena Dubal investigated the anti-ageing effect of injecting mice with the enzyme klotho, which had previously been identified as an anti-ageing factor[11]. They found that klotho triggered the animals’ own production of PF4, which then permeates the brain and enhances cognition.
Dr Odette Leiter and Dr Tara Walker's #research has discovered that platelets, the tiny blood cells critical for blood clotting, secrete a protein that rejuvenates #neurons in aged mice in a similar way to physical #exercise.
— Queensland Brain Institute, UQ (@QldBrainInst) August 17, 2023
Read more:
🔗 https://t.co/Z5IZx71dBk pic.twitter.com/yAINIDckkl
However, the results suggest that other platelet factors may also contribute to this beneficial effect. Meanwhile, Odette Leiter from the University of Queensland at Brisbane, Australia, and colleagues studied rejuvenating effects of exercise, looking for molecular factors that mediate these effects, so called exerkines[10]. They homed in on one specific exerkine, which also turned out to be PF4, suggesting that this protein is an important point in the maintenance of youthful brain function, where several pathways converge.
References
- J. Johansen et al., Nature Microbiol., 2023, 8, 1064; doi: 10.1038/s41564-023-01370-6
- Lei Zhang et al., Sci. Adv., 2023, 9, eadg7056; doi: 10.1126/sciadv.adg7056
- J. R. Poganik et al., Cell Metabol; doi: 10.1016/j.cmet.2023.03.015
- C. A. Kern et al., Nature Commun. 2023, 14, 4381; doi: 10.1038/s41467-023-40088-1
- C. A. Kern, D. Gems, Front. Genet., 2022; doi: 10.3389/fgene.2022.880343
- A. A. Ruparelia et al., Aging Cell, 2023, e13862; doi: 10.1111/acel.13862
- P. Singh et al., Science, 2023, 380, eabn9257; doi: 10.1126/science.abn9257
- Z. Zhou et al., Science, 2023, 380, 376; doi: 10.1126/science.add7631
- J.-H. Yang et al., Aging-US, 2023, 15, 5966; doi: 10.18632/aging.204896
- A. B. Schroer et al., Nature 2023, 620, 1071; doi: 10.1038/s41586-023-06436-3
- C. Park et al. Nat. Aging 2023, 3, 1067; doi: 10.1038/s43587-023-00468-0
- O. Leiter et al. Nat. Commun. 2023, 14, 4375; doi: 10.1038/s41467-023-39873-9






