The promising properties of metal halide perovskites include their high light-harvesting capacity and their remarkable ability to convert solar energy into electrical energy, Maria Burke reports
Image caption: Just adding a bulky molecule to the surface of a perovskite might finally make the material stable enough for incorporating into solar panels. Credit: Purdue University illustration/Enzheng Shi.
Perovskites are under development as alternatives to silicon in thin-film solar cells. The power conversion efficiency of perovskite solar cells (PSCs) has increased from 3.8% to 25.5% in only ten years, surpassing other thin-film solar cells and catching up with state-of-the-art polycrystalline silicon. While silicon remains the dominant material used in solar cells, it’s approaching its theoretical maximum energy efficiency of 29.1%, with a record 26.7% established to date.
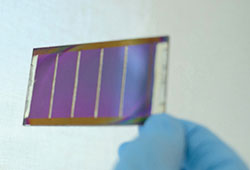
Perovskites have the potential to be even more efficient than silicon because less energy is wasted when converting solar energy to electricity. They also create lightweight, flexible and semi-transparent cells. As they can be processed from a solution at moderate processing temperatures into a thin film, like ink printed on paper, they could be more cheaply produced, and in higher quantities, than silicon. Perovskite technologies are progressing rapidly towards commercialisation, as researchers work on a few remaining challenges such as stability and scalability.
Named after a naturally occurring mineral that shares a structurally similar chemical formula, perovskites are synthetic composites that have 3D lattice crystal structures made up of organic materials bound to a metal. As well as showing potential for use in solar cells, they could also help make optoelectronic devices such as lasers and light-emitting diodes (LEDs) more efficient.
Hybrid halide perovskites have attracted considerable attention. They have the structure ABX3 where A stands for a cation, either an organic molecule or some alkali metal; B is a metal, often lead; and X is a halide element such as bromide or iodide. A good organic example is methylammonium lead iodide or bromide. It has high conversion efficiencies, but is not very stable. Inorganic perovskites with caesium at the A-site promise higher stabilities, but some do not provide the electronic properties needed for applications in solar cells or other optoelectronic devices.
Hybrid halide perovskites have the structure ABX3 where A stands for a cation, either an organic molecule or some alkali metal; B is a metal, often lead; and X is a halide such as bromide or iodide.
29%
Silicon remains the dominant material used in solar cells but is approaching its theoretical maximum energy efficiency of 29.1%, with a current record of 26.7%.
27%
Combining a triple halide perovskite with silicon boosts efficiency to 27%, compared with 21% for a pure silicon cell.
1/5th
Illumination is responsible for around a fifth of global electricity consumption, a figure that could be reduced to 5% if all light sources consisted of LEDs.
Instability
The most important obstacle to widespread use of PSCs is their poor stability under real world conditions. One particular perovskite – FAPbI3 (formamidinium lead iodide) – has been shown to be an ideal candidate for efficient, stable PSCs, but only at high temperatures. At normal temperatures below 150°C, FAPbI3 undergoes a phase transition from a black photoactive phase to a yellow phase that does not react to light, making it useless as a solar cell. Previously, researchers have tried to overcome this problem by mixing it with other ions such as caesium or bromide.
Taking a different approach, researchers Michael Grätzel and Anders Hafgeldt at Ecole Polytechnique Fédérale de Lausanne, Switzerland, have tweaked the usual deposition method of manufacturing PSCs.1 They treated the materials with methylammonium thiocyanate or formamidinium thiocyanate vapour before deposition. This converted the yellow phase to the desired pure black phase, and it remained black after 500 hours at 85°C. When the team used the new FAPbI3 films to make PSCs, the cells showed more than 23% power-conversion efficiency, and long-term operational and thermal stability.
Meanwhile, an international team, involving Dutch, US and Chinese researchers, has been investigating the performance of PSCs based on formamidinium-caesium lead iodide (FACsPbI3) over a range of long-term operating conditions. They have been looking at what causes degradation to happen in the first place.2 They conclude that sunlight generates charged particles in the solar panel, which tend to flow to places where the band gap – the minimum amount of energy needed for generating the free electrons – is lowest, in this case the formamidinium perovskite. The resulting energy differences make the mixed compounds, which worked together so well to make the cell efficient, fall apart into separate clusters. It appears that especially the caesium-heavy clusters become photo-inactive and block current flow, limiting the performance of the device.
According to Shuxia Tao of Eindhoven University of Technology in the Netherlands, the new findings are one step further to finding the way to possible solutions. ‘We were able to thoroughly understand the instability of halide perovskites that are intrinsic to device operation. This opens the possibility for designing new perovskite compositions for the ultimate stable solar cells.’ Possible strategies include using additives to enhance the chemical interactions inside the materials in the panels, tuning the band gaps by using other elements like bromide and rubidium instead of iodide and caesium, or modifying the energy levels to extract photo-carriers more efficiently.
Researchers at the US Department of Energy’s National Renewable Energy Laboratory (NREL) and the University of Colorado, Boulder, US, have also been looking at ways to improve the longevity and efficiency of PSCs. In particular, they wanted to tackle a problem called light-induced phase-segregation, which occurs when the alloys that make up the solar cells break down under exposure to continuous light.
PSCs usually consist of a combination of iodine and bromine, or bromine and chlorine, but the NREL researchers found that including all three types of halides improved stability. In this study, they added chlorine to iodine and bromine to create a triple-halide perovskite phase.3 They found that this suppressed the light-induced phase-segregation even at an illumination equivalent to 100 suns. The new formula created a solar cell with an efficiency of 20.3%. Significantly, there was minimal degradation: less than 4% after 1000 hours of operation at 60°C. At 85°C and after operating for 500 hours, the solar cell lost about 3% of its initial efficiency.
‘Now that we have shown that we are immune to this short-term, reversible phase-segregation, the next step is to continue to develop stable contact layers and architectures to achieve long-term reliability goals, allowing modules to last in the field for 25 years or more,’ says NREL’s Caleb Boyd. ‘The next step is to further demonstrate accelerated stability testing to really prove what might happen in 10 or 20 years in the field.’
This team has also been experimenting with ‘tandem’ cells, combining perovskites with silicon to boost efficiency and reduce the costs associated with solar electricity. Their tandem cell contained a triple halide perovskite as the top cell. It had a reported efficiency of 27%, compared with 21% for a pure silicon cell. Using two active layers means the solar spectrum is absorbed more completely, they explain, provided that the two cells are matched appropriately.
Layer stability
Another approach to increasing stability involves creating layers of different perovskites. A Purdue University-led US research team has found a way to make halide perovskites that inhibit the ion movement that makes them rapidly degrade, unlocking their use for solar panels as well as electronic devices.4 The discovery also means that halide perovskites can stack together to form heterostructures that would allow a device to perform more functions.
‘If an engineer wanted to combine the best parts about perovskite A with the best parts about perovskite B, that typically can’t happen because the perovskites would just mix together,’ says Brett Savoie of Purdue University.
But the team found that by adding a rigid bulky molecule called bithiophenylethylammonium to the surface of a perovskite stabilises the movement of ions, preventing chemical bonds from breaking easily. And it remains stable even when heated to 100°C. ‘In this case, you really can get the best of A and B in a single material. That is completely unheard of.’
The researchers also demonstrated that adding this molecule makes a perovskite stable enough to form clean atomic junctions with other perovskites, allowing them to stack and integrate, which has previously proved challenging.
The attraction of using multiple perovskites in a solar cell – or integrated circuit – is that it would allow the device to perform different functions. For example, a pattern of perovskites incorporated into a computer chip might allow it to do more than just one material.
LEDs and lasers
Research is also focusing on using perovskites in electronic devices. For example, Feng Gao’s team at Linköping University in Sweden has developed efficient blue light-emitting diodes (LEDs) based on halide perovskites, which show potential for cheap and energy-efficient illumination and monitors.5 Illumination is responsible for around a fifth of global electricity consumption, a figure that could be reduced to 5% if all light sources consisted of LEDs, the researchers say. The blue-white LEDs currently in use, however, need complicated manufacturing methods and are expensive.
While researchers have used perovskites to make LEDs for green and red light, it’s proved much harder for blue light. But blue light is the key to bringing light-emitting perovskites to practical applications. ‘Metal-halide perovskites are easily colour-tuneable over the whole visible spectrum by simple alloying,’ explains researcher Max Karlsson. ‘Unfortunately, they exhibit de-mixing and a blue LED turns green during operation. We have now found a method that can prevent this colour shift by controlling the film crystallisation dynamics when creating the perovskite.’
The challenge of creating blue light in perovskites is that it requires a chemical composition with a large fraction of chloride, which makes the perovskite unstable. Blue perovskite-based LEDs have previously been created using the ‘quantum confinement technique’, which gives low-intensity LEDs with poor efficiency. This new study uses ‘vapour-assisted crystallisation’.
The team made halide perovskites which emitted light in the wavelength range 451 to 490nm – corresponding to deep blue to sky blue colours. They report their LEDs were both stable and efficient with an energy efficiency of up to 11%, claimed as one of the most efficient blue perovskite-based LEDs developed.
These findings pave the way for stable perovskite alloys, not only for LEDs but also for solar cells. However, more work is needed before perovskite LEDs can compete with existing technologies, such as those based on semiconductors, in terms of lifetime and performance.
Lasers are another area where researchers are investigating the potential of perovskites because they can be fabricated cheaply to have tuneable colours. However, lasers made from perovskites suffer from a phenomenon called lasing death, which causes lasing to stop after a few minutes at room temperature.
Now an international team of researchers, led by Kyushu University in Japan and Changchun Institute of Applied Chemistry (CIAC) in China, has produced a perovskite-based laser that operated for over an hour at room temperature.6 To overcome lasing death, they focused on energetic states called triplet excitons. Opposite charges in electronic devices can temporarily form an energetic state called an exciton, which can exist as singlets or triplets. Triplet exitons can’t emit light.
The team found evidence of triplet exitons with long lifetimes – nearly one microsecond – in the perovskites they had made. ‘Triplets do not emit light and have a tendency to interact with light-emitting singlets in a way that causes both to lose their energy without producing light,’ explains Chuanjiang Qin of CIAC. ‘Thus, if triplets are present in perovskites, we likely need to get them out of the way so they do not interfere with lasing.’
To do this, the researchers incorporated an organic layer into the perovskites to hold triplets in a low energy state. Triplet excitons transfer from the light-emitting portion of the perovskite to the organic layer, which appears to allow lasing to continue without interruption. Another solution was to place the perovskite layer of the laser in air, as oxygen destroys triplets. These new findings will pave the way for the future development of a new class of electrically operated lasers based on perovskites that are low cost and easily fabricated, Qin says.
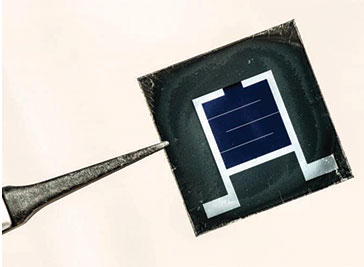
Images above: The coloured semi-transparent version of a perovskite solar mini module achieved similar measures of power conversion efficiency across a whole range of different colours. Credit: NTU Singapore
Scalability issues
Another challenge facing development of perovskite solar cells (PSCs) is scaling up what works in the lab to what works on a large-scale in the real world. Researchers at Singapore’s Nanyang Technological University recently demonstrated highly efficient large-area PSCs made using an industrially compatible process. They claim to have created one of the highest power conversion efficiencies of any perovskite-based device larger than 10cm2.
The team adapted an industrial coating technique used to produce electronics called thermal co-evaporation and found that it could fabricate solar cell modules of 21cm2 with power conversion efficiencies of 18.1%, one of the highest recorded values reported for scalable PSCs.7
‘The best-performing perovskite solar cells have so far been realised in the laboratory at sizes much smaller than 1cm2, using a solution-based technique called spin-coating,’ explains Annalisa Bruno, lead author. ‘However, when used on a large surface, the method results in perovskite solar cells with lower power conversion efficiencies. This is due to the intrinsic limitations that include defects and lack of uniformity over large areas, making it challenging for industrial fabrication methods.’ By using thermal evaporation to form the perovskite layer, the team got around some of these issues.
Using the same technique, the researchers then fabricated coloured semi-transparent versions of the PSCs and mini modules, which achieved similar measures of power conversion efficiency across a whole range of different colours. The team suggests the solar mini modules could be used on facades and windows in skyscrapers, which is not possible with current silicon solar panels as they are opaque and block light. They are also looking at integrating perovskite and silicon solar cells to create a ‘tandem’ solar cell.
Top: Perovskites can be used to create light-weight flexible and semi-transparent solar cells ideal for applications in buildings and a variety of urban spaces.
Above: The coloured semi-transparent version of a perovskite solar mini module achieved similar measures of power conversion efficiency across a whole range of different colours.
References
1 M. Graetzel et al, Science, doi: 10.1126/science.abb89852 H. Zhou et al, Joule, doi: 10.1016/j.joule.2020.06.005
3 M.D. McGehee et al, Science, doi: 10.1126/science.aaz5074
4 L. Dou et al, Nature, doi: 10.1038/s41586-020-2219-7
5 F. Gao et al, Nature Commun., doi: 10.1038/s41467-020-20582-6
6 C. Qin et al, Nature, doi: 10.1038/s41586-020-2621-1
7 A. Bruno et al, Joule, doi: 10.1016/j.joule.2020.03.005