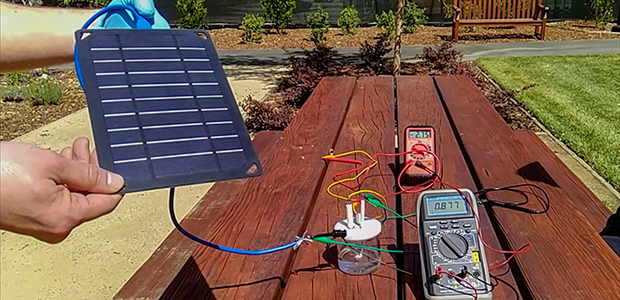
New hydrogen fuel has been generated using solar power, electrodes and sea water by researchers in Stanford, US.
Cassie Sims
Hydrogen fuel is seen as a green source of energy that could help to replace carbon-based fuels, which have a negative environmental impact. Hydrogen gas can be generated simply by ‘splitting’ water, but current methods require highly purified water. New research has found a way to produce hydrogen gas using sea water.
‘Hydrogen is an appealing option for fuel because it doesn't emit carbon dioxide,’ said Hongjie Dai, Professor of Chemistry at Stanford University. ‘Burning hydrogen produces only water and should ease worsening climate change problems.’
Currently, we would need very large amounts of purified water to produce enough hydrogen to power large infrastructure and household vehicles. Highly purified water is expensive to produce and is a precious resource.
Hydrogen gas is produced from water by electrolysing it with two simple electrodes. When electricity is passed through the water, the water molecules ‘split’ producing hydrogen bubbles at the negative end, and oxygen bubbles at the positive end.
Using saltwater causes corrosion due to the chloride ions it contains. This significantly limits the lifespan of the equipment. Scientists have discovered that by covering the negative electrode with layers rich in negative charges, they repel the negatively charged chloride ions and reduce decay of the metal.
A prototype of the hydrogen-generating device, which is also solar powered. Image: H. Dai, Yun Kuang and Michael Kenney
Without the coating, the electrode only lasts about 12 hours in sea water. However, by adding the layers they can go for more than a thousand hours. Previous solutions to the problem involved reducing the electric currents used to much lower levels.
‘The impressive thing about this study was that we were able to operate at electrical currents that are the same as what is used in industry today,’ said Michael Kenney, a graduate student in the Dai lab and co-lead author.
The technology has the potential to be scaled up and used to generate hydrogen gas for fuel or producing breathable oxygen directly from seawater for divers and submarines.
Related Links: