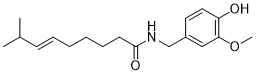
Do you know how the Academy Awards came to be named the Oscars? What about the story behind the Nobel prize? Behind every award name there is a story, and the Julia Levy Award is no exception.
On the face of it, the Julia Levy Award is about innovation in biomedical applications, but it is the stories of the winners of this SCI Canada award, and Julia Levy herself, that really give it life.
But for a tweak of history, Julia Levy may not have ended up in Canada at all. Born Julia Coppens in Singapore in 1934, she moved to Indonesia in her early childhood. Her father uprooted the family during the Second World War and she left for Vancouver with her mother and sister – her father only joining them after release from a Japanese prisoner-of-war camp.
Julia and her family moved to Vancouver during the Second World War.
After studying bacteriology and immunology at the University of British Columbia (UBC), the young Julia received a PhD in experimental pathology from the University of London. She went on to become a professor at UBC and helped found biopharmaceutical company Quadra Logic Technologies in 1984.
More important than confining her achievements in cold prose, Julia Levy’s work made a profound difference to people’s lives. She developed a groundbreaking photodynamic therapy (PDT) that treated age-related macular degeneration – one of the leading causes of blindness in the elderly. She also created a bladder cancer drug called Photofrin in 1993 and, according to Neil and Susan Bressler, the Visudyne PDT treatment created by Julia and her colleagues was the only proven treatment for certain lesions.
Levy thrived in the business space too, serving as Chief Executive Officer and President of QLT from 1995 to 2001. She has since won a boatload of awards for her achievements, but sometimes the best testimonies come from those who have been inspired by her achievements.
Trailblazing drug discovery systems
For Helen Burt, winner of the 2022 Julia Levy Award and retired Angiotech Professor of Drug Delivery at the University of British Columbia (UBC), Julia has been an inspiration. Here was this UBC professor who jointly founded this big, exciting company – creating medication that improved people’s lives and showing her what was possible.
Helen, an English native, moved to Vancouver in 1976 for her PhD and loved it so much that she stayed. As a professor at UBC, Helen would become a trailblazer in drug delivery systems – a field pioneered earlier by Julia Levy.
‘I was a new assistant professor when she was building Quadra Logic and I would go to talks that she gave,’ Helen said. ‘Essentially, the early technology for QLT was a form of very sophisticated drug delivery [...] It was getting the drug they developed into the eye and irradiating it with light of a specific wavelength.
‘It was very, very targeted. And so, you didn’t get the drug going elsewhere in the body and causing unwanted side effects. So her technology was a form of very advanced drug delivery technology.’

‘For me to win an award that honours Julia Levy and her achievements – I think that's what makes it so special to me.’ – Professor Helen Burt, a former student of Julia Levy, is the Award's most recent recipient.
>> Learn more about SCI Canada.
These talks chimed with the young Helen. If a microbiologist could develop this kind of technology, what was stopping her from developing her own?
She, too, became a pioneer in her field, developing nanoparticle-based drug delivery systems (including those to treat cancer) and a novel drug-eluting coronary stent. According to Professor Laurel Schafer, who put Helen forward for the Julia Levy Award: ‘[Helen] was a trailblazer in new approaches for drug delivery and in research leadership on our campus.’
Importance to Canadian chemistry
Professor Schafer is a hugely accomplished chemist in her own right; and the University of British Columbia chemistry professor’s achievements in catalysis discovery were recognised with the LeSueur Memorial Award at the 2020 Canada Awards.
Julia Levy provided an inspiration to Laurel too, in her case as an exemplar for what Canadian chemists could achieve. ‘The achievements of Julia Levy show that it really can be done right here in Canada, and even right here in British Columbia,’ she said. ‘I grew up in a Canada where I believed that better was elsewhere and our job was to attract better here – a very colonial attitude.

Julia studied at and later became a Professor at the University of British Columbia – the campus is pictured above.
‘I now believe and know that better is right here. Professor Levy’s work showed that world-leading contributions come from UBC and from the laboratories led by women.’
She noted that the Julia Levy Award acknowledges Canadian innovation in health science, whereas Canadian chemistry has historically focused on process chemistry in areas such as mining and petrochemicals.
But Julia Levy’s influence permeates beyond science. ‘Julia is one of those people who has been willing throughout her whole career – even now, well into her eighties – to give back to the community,’ Professor Burt says. ‘She mentors, she coaches, she sits on the boards of startup companies, and she advises.’
‘She’s just got this incredible amount of knowledge… She was the Chief Executive Officer [at QLT], so she learnt all of the aspects: the complex and sophisticated regulations, knowing how to find the right people to conduct clinical trials, and how to do the scale-up. She really is a legend in terms of giving back to the community. And this is not just in British Columbia – it’s Pan-Canadian.’

Pictured above: Julia Levy
For young chemists, the Julia Levy in the Julia Levy Award may just be a name for now, but for those in the Canadian chemical industry and patients all over the world, her influence and her work resonate.
As Professor Helen Burt said: ‘For me to win an award that honours Julia Levy and her achievements – I think that's what makes it so special to me.’
>> For more information on the Canada Awards, go to: https://bit.ly/3VMwNKa
What makes chilli peppers so spicy and how do they help with pain relief? The SCI Horticulture Group explained all ahead of their appearance at BBC Gardeners’ World Live in Birmingham from 16-19 June.
This June, the SCI Horticulture Group will tell the public all about the hidden chemistry behind their favourite fruit and vegetable plants. One of the main plants they will feature at the National Exhibition Centre is the humble chilli pepper – and these famous fruit-berries conceal more secrets than you might think…
Where does the chilli originate?
The chilli pepper (Capsicum spp.) is a member of the Solanaceae, the plant family that includes edibles such as potatoes, tomatoes, aubergines, but also poisonous plants such as tobacco, mandrake, and deadly nightshade.
Who brought the chilli pepper to these shores?
The chilli was brought to Europe in the 15th century by Christopher Columbus and his crew. They became acquainted with it on their travels in South and Central America and, shortly thereafter, to India via the Portuguese spice trade.
How varied is the genus?
Of the 42 species in the capsicum genus, five have been domesticated for culinary use. Capsicum annuum includes many common varieties such as bell (sweet) peppers, cayenne and jalapenos. Capsicum frutescens includes tabasco. Capsicum chinense includes the hottest peppers such as Scotch bonnet. Capsicum pubescens includes the South American rocoto peppers, and capsicum baccatum includes the South American aji peppers.
From the five domesticated species, humans have bred more than 3,000 different cultivars with much variation in colour and taste. The chilli and bell peppers that we eat are the fruit – technically berries – that result from self-pollination of the flowers.
>> The SCI Horticulture Group brings together those working on the wonderful world of plants.
And we eat a lot of them?
Today, chilli peppers are a global commodity. In 2019, 38 million tonnes of green chilli peppers were produced worldwide, with China producing half of the total. Spain is the largest commercial grower of chillies in Europe.
Capsaicin helps give chilli peppers their heat
What makes chillies hot?
Capsaicin is the main substance in chilli peppers that provides the spicy heat. It binds to receptors that detect and regulate heat (as well as being involved in the transmission and modulation of pain), hence the burning sensation that it causes in the mouth.
In humans, these receptors are present in the gut as well as the mouth (in fact, throughout the peripheral and central nervous systems) – hence the after-effects of eating too much chilli. Capsaicin, however, is not equally distributed in all parts of pepper fruit. Its concentration is higher in the area surrounding the seeds.
>> Get tickets for Gardeners’ World Live 2022 and pop by our stand to say hello!
How do you measure chilli heat?
The Scoville Heat Unit Scale is used to classify the strength of chilli peppers. Scoville heat units (SHU) were named after American pharmacist Wilbur Scoville who devised a method for rating chilli heat in 1912.
The ludicrously hot Dragon’s Breath chilli
This method relied on a panel of tasters who diluted chilli extract with increasing amounts of sugar syrup until the heat became undetectable. The greater the dilution to render the sample’s heat undetectable, the higher the SHU rating. Pure capsaicin measures 16,000,000 SHU.
The capsaicin content of chilli peppers varies wildly, as is reflected in the SHUs of the peppers below:
- Bell (Sweet peppers) = 0 SHU
- Jalapeno = 5,000 SHU
- Scotch Bonnet = 100,000 SHU
- Naga Jolokia = 1,040,000 SHU
- Carolina Reaper = 1,641,183 SHU
- Dragon’s Breath = 2,480,000 SHU
Dispersal vs. protection – why do chillies contain capsaicin?
The seeds of chillies are dispersed in the wild by birds who do not have the same receptors as mammals and, therefore, are unaffected by capsaicin. Perhaps chillies have evolved to prevent mammals from dispersing their seeds?
Capsaicin has also been shown to protect the plant against fungal attack, thus helping the fruit to reach maturity and the seeds to be dispersed before succumbing to rot. This antifungal property can also be put to good use in helping to preserve foods for human consumption.
How did chilli help win a Nobel Prize?
Capsaicin was pivotal in the research that led to the award of the 2021 Nobel Prize in physiology and medicine to David Julius and Ardem Patapoutian for their discoveries of receptors for temperature and touch.
The two US-based scientists received the accolade for describing the mechanics of how humans perceive hot, cold, touch, and pressure through nerve impulses. The research explained at a molecular level how these stimuli are converted into nerve signals, but the starting point for the study was work with capsaicin from the humble chilli pepper.
Capsaicin in medicine
Capsaicin is used as an analgesic (a pain reliever) in topical ointments, nasal sprays, and patches to relieve chronic and neuropathic pain. Clinical trials continue to investigate the potential of capsaicin for a wide range of additional pain indications and as both an anti-cancer and anti-infective agent.
>> Special thanks to Neal Price from Chillibobs, Martin Peacock of ZimmerPeacock, Hydroveg, and The University of Reading Soft Fruit Technology Group for supporting the work of the SCI Horticulture Committee at BBC Gardeners’ World Live.
>> Our resident gardening expert, Geoff Dixon, provides plenty of gardening tips on the SCIBlog.
From genome mining and green synthesis, to tackling tuberculosis and computational methods to help cure malaria, the chemists of tomorrow have been busy showcasing their talents as part of the Society of Chemical Industry Young Chemists Panel’s National Undergraduate Online Poster Competition 2021.
A snapshot of these students’ talents is bottled below in their own words. So, which one of these 15 entries do you think contains the most potential?
1: Genome mining for myxobacterial natural products discovery
Emmanuelle Acs et al., University of Glasgow
Natural products have always had a privileged place in drug development programmes, but their discovery is long and tedious. Genome mining arises as a solution allowing the finding of compounds never seen before. Using an array of bioinformatic softwares, the myxobacterial genome was explored for new Ribosomally and Post-Translationally modified Peptides (RIPPs). Myxobacteria are soil-dwelling bacteria known for the number of secondary metabolites they produce, and they have proven to hide many more within their genome. Indeed, our analyses have led to the potential discovery of nine new myxobacterial natural products. The nature and class of these products is to be confirmed by biosynthesis in the laboratory.
2: De novo design of a lanthanide-binding peptide inspired by the methanol dehydrogenase enzyme active site
Olivia Baldwin et al., University of Birmingham
Lanthanides were thought to be biologically irrelevant until the discovery of bacteria containing the lanthanide-dependent methanol dehydrogenase (Ln-MDH) enzyme. There has been interest in exploiting the attractive properties of the lanthanides by the de novo design of artificial proteins, aiming to explore protein structures and functions not observed in biology. Here, a lanthanide-binding peptide, CS1-0, has been designed de novo and shown to bind to europium and pyrroloquinoline-quinone (PQQ), a key component of the Ln-MDH active site. This partial recreation of a biologically relevant lanthanide binding site is a step towards the ultimate goal of de novo design, to create functional artificial metalloproteins with simplified structures.
3: Template-directed synthesis of porphyrin nanorings: a computational and experimental study
Janko Hergenhahn et al., University of Oxford
Template-directed synthesis provides a route to achieve porphyrin nanorings by favouring ring-closure reaction over oligomerisation. A structurally new template with 12 binding sites has been proposed for the synthesis of novel porphyrin rings; however, initial unsuccessful reactions have raised questions about the binding efficiency of this template to the linear substrate. We have employed classical and quantum modelling together with experimental techniques to explore template-substrate binding in solution and shed light on this process. Titration experiments and modelling have enabled us to study the occupancy of different binding sites and quantify the influence of strain on binding, further guiding novel designs.
4: Transborylation enabled boron-catalysed hydrocyanation of enones
Kieran Benn et al., University of Edinburgh
Hydrocyanation offers an orthogonal route to synthetically ubiquitous amines. Current hydrocyanation methodologies are dominated by the use of acutely toxic hydrogen cyanide gas and transition metal catalysts. Here the application of main-group catalysis and transborylation is reported for the formal hydrocyanation of functionalised alkenes. The catalytic protocol was optimised and applied to a broad range of substrates (20 examples), including examples where chemoselectivity was demonstrated in the presence of reducible functionalities and Lewis basic groups. Mechanistic studies support a proposed catalytic cycle in which B–N/B–H transborylation was a key to catalyst turnover.
Students at the University of Glasgow have used computational analysis to help tackle malaria.
5: Investigation of the structure-property relationship between hydrophobicity and the antimicrobial activity of AMP-mimic copolymers
Xiyue Leng et al., University of Birmingham
Antimicrobial peptides are increasingly employed as new-generation antibiotics, with their amphiphilic nature (contain both hydrophobic and cationic components) mimicked by polymers to enable a more cost-effective approach. However, there is a lack of a quantitative pre-experiment indicator to provide a prospect on their potency. The overall hydrophobicity represented by LogP/SA was proposed to rapidly identify candidates in future designing to reduce synthetic efforts. We show a comparison study between two computational tools used to calculate LogP/SA: ChemBio3D and Materials Studio, in terms of the predictive power and sensitivity, followed by the synthesis of copolymers with a different cationic side chain based on the calculation results.
6: Modelling potential SARS-CoV-2 VIRTAC warheads
Mirjam-Kim Rääbis et al., University of Glasgow
Traditional small molecule therapeutics in medicinal chemistry often require high doses to inhibit the target protein, leading to issues with safety and drug resistance. Proteolysis targeting chimeras (PROTACs) are a new class of molecule that combat these issues, as they can use the body’s own protein degradation systems to degrade targets even at low drug doses. Virus-targeting chimeras (VIRTACs) can use a similar mechanism to target viral proteins. This project uses molecular docking studies to explore potential VIRTAC warheads that target the papain-like protease of SARS-CoV-2, in an attempt to find a potential treatment to COVID-19 that would, among other benefits, offer a lower risk of antiviral resistance.
7: Design, synthesis and biological evaluation of anti-tubercular small molecules
Miriam Turner et al., Newcastle University
Tuberculosis remains one of the top 10 causes of death worldwide, therefore there exists an unmet clinical need for new and improved therapeutics that tackle increasing bacterial resistance and affordability issues. Previous studies indicate N-substituted amino acid hydrazides exhibit good activity against several strains of Mtb. Ongoing structure-activity relationship studies utilising isoniazid, a variety of amino acids, and the active imidazo[1,2-a]pyridine-3-carboxy moiety of clinical candidate Q203 have also demonstrated excellent activities. Herein we report the results of our continued evaluation of this architecture, using a scaffold hopping approach to explore the potential of this pharmacophore as a new anti-tubercular drug.
8: Towards a cure for malaria: computational analysis and design of PfCLK3 inhibitors
Skye Brettell et al., University of Glasgow
Malaria continues to pose a significant challenge to humanity. Resistance to several frontline antimalarials represents a considerable threat, marking the need for new drugs with novel mechanisms of action. Kinase inhibitors represent a potential new class of antimalarials. TCMDC-135051 is a hit compound with activity against malarial kinase PfCLK3 as well as potency in liver, blood and sexual stage parasites. During this project, sequential analysis of the PfCLK3 catalytic domain identified key structural differences between the target and its human orthologs. Molecular docking studies of TCMDC-135051 analogues using GOLD then yielded potential lead compounds with predicted high affinity for the target kinase.
9: Mind the gap! Through-space electronic stabilisation of dynamic azaacene-extended helicene diimides
Matteo Albino et al., University of York
The strain-induced contortion of non-planar, chiroptically-active helicenes caused by fjord steric repulsive interactions is well known. Fjord-mediated planarisation, on the other hand, is far less common and has typically only been achieved via inherently strong covalent bond formation. Herein, I present the properties and density functional theory (DFT) analysis of electroactive aza[5]helicenes exhibiting unexpected through-space π-electronic stabilisation in the reduced states as a result of non-covalent fjord bonding effects. Computational modelling of optical spectra and aromatic-induced current densities reveal that lone pair-repulsive nitrogens in the fjord promote favourable ring currents and reversible helicene planarisation.
10: Mitochondria targeted metal-chelators as potential therapeutic agents against Parkinson’s Disease
Sam Andrew Young et al., Northumbria University
The synthesis of metal chelating molecules, specifically hydroxypyridones (HOPOs), have been identified as potential therapeutic agents for treating Parkinson’s Disease (PD) as bidentate ligands at the two oxygen donor atoms. These ligands are selective for ferric iron in the body, which is expected to stop the reduction of this iron accumulated in the brains of PD sufferers, hindering the Haber-Weiss mechanism from taking place in the mitochondria of the cell and preventing the associated degeneration of the cells. The lipophilicity of these HOPOs is vital to the process, allowing the molecule to transverse the blood-brain barrier, the addition of a triphenylphosphonium group on the HOPO is thought to increase therapeutic effect.
At Heriot Watt University, students have investigated the skin irritation potential of nanoclays using an IATA
11: Green synthesis of n-acetylcolchinol
Adelaide Lunga et al., Loughborough University
The aim of this project is to develop a short synthesis of N-acetylcolchinol using a greener and step-economical pathway. First, aldol condensation of 3-hydroxyacetophenone and 3,4,5-trimethoxybenzaldehye using ethanolic NaOH produced the respective chalcone. The product was reduced electrochemically in DMSO:MeOH (4:1) employing carbon electrodes and NEt4Cl to the saturated benzylic alcohol, which was converted to an acetamide via Ritter reaction using H2SO4 in MeCN. In the final step, the conditions were optimised to enable electrochemical oxidative coupling of the aromatic groups to give the desired N-acetylcolchinol. This novel four-steps reaction sequence avoids use of transition metal catalysts or toxic reagents.
12: Towards the synthesis of small-molecule probes to measure endosulfatase activity
Yi Xiao et al., University of Oxford
Human endosulfatases (SULFs) are enzymes on the cell surface and in the extracellular matrix that hydrolyse 6-O-sulfate on glucosamine units within heparan sulfate proteoglycans. SULFs are involved in growth and development, muscle regeneration and tumour growth via various signaling pathways, with untapped therapeutic and diagnostic potentials. However, profiling SULFs remains a challenge. Antibodies detect their presence, but do not indicate their activity state. The current activity assay is a global sulfatase assay and is not selective in a biological sample. We propose a novel small-molecule probe to profile SULF activity by exploiting the formation of 1,6-anhydrosugar, which can be potentially used in isolated proteins and in vitro.
13: Developing machine learning models to predict the Hansen solubility parameters
Alexander Pine et al., University of Greenwich
Solubility parameters are important for pharmaceutical formulations, paint formulations and new material development. There is a need to improve the accuracy of solubility calculations, and to be able to make rapid predictions of the solubility of new molecular structures. In this project, a range of Python plugins, and open-source codes have been used to develop a Lasso linear regression machine learning model to predict the Hansen solubility parameters (HSP) - δd, δp and δh, which represents dispersion forces, dipole-permanent dipole forces and hydrogen bonding respectively with the intention of making faster and more accurate prediction in solubility.
14: The cycloaddition between cyclononyne and mesyl azide
Alexander David Robertson et al., The University of Glasgow
This research considers computational modelling of a SPAAC reaction involving cyclononyne. DFT calculations were performed on the strain promoted reaction between cyclononyne and mesyl azide. Three low energy conformers of cyclononyne with Cs, C2 and C1 symmetry were found with similar energy. The transition structures for the corresponding cycloaddition with mesyl azide were found and the C2 conformer was the lowest in energy. Product structures were found leading to the identification of the thermodynamic product of the reaction. Distortion/interaction analysis showed that the cycloalkyne was already significantly pre-constrained to its reacting geometry.
15: The assessment of skin irritation potential of nanoclays using an IATA
Holly King et al., Heriot Watt university
Clays are natural nanomaterials consisting of mineral silicate layers. They have several functional uses in everyday life. An example of nanoclays that carry out a wide range of roles is smectites which include montmorillonite (MMT), bentonite and hectorite. These nanoclays can be used in cosmetics, altering their appearance and in pharmaceuticals as drug carriers and wound dressings. Integrated approach to testing and assessment (IATA) aim to collect all relevant data into one easy to understand format that can be used to group materials. Using an IATA dedicated to skin irritation/corrosion it was found that MMT was safe for use. However, hectorite was found to be toxic at high doses indicating that it is a possible irritant to the skin.
Many thanks to the sponsors of this year’s competition: GSK, AstraZeneca, TeledyneIsco. The event runs until 9 July, so let us know what you think of the entries on Twitter at #SCIPosterComp.
If you’d like to see these students’ full posters, go to: https://istry.co.uk/postercompetition/5/?date_example=2021-06-28
Many of us have contemplated buying a reconditioned phone. It might be that bit older but it has a new screen and works as well as those in the shop-front. I’m not sure, however, that any of us have thought of investing in a reconditioned liver – but it could be coming to a body near you.
Researchers based in São Paulo’s Institute of Biosciences have been developing a technique to create and repair transplantable livers. The proof-of-concept study published in Materials Science and Engineering by the Human Genome and Stem Cell Research Centre (HUG-CELL) is based on tissue bioengineering techniques known as decellularisation and recellularisation.
The organs of some donors are sometimes damaged in traffic accidents, but these may soon be transplantable if the HUG-CELL team realises its goal.
The decellularisation and recellularisation approach involves taking an organ from a deceased donor and treating it with detergents and enzymes to remove all the cells from the tissue. What remains is the organ’s extracellular matrix, containing its original structure and shape.
This extracellular matrix is then seeded with cells from the transplant patient. The theoretical advantage of this method is that the body’s immune system won’t rile against the new organ as it already contains cells from the patient’s own body, thereby boosting the chance of long-term acceptance.
However, the problem with the decellularisation process is that it removes the very molecules that tell cells to form new blood vessels. This weakens cell adhesion to the extracellular matrix. To get around this, the researchers have introduced a stage between decellularisation and recellularisation. After decellularising rat livers, the scientists injected a solution that was rich in the proteins produced by lab-grown liver cells back into the extracellular matrix. These proteins then told the liver cells to multiply and form blood vessels.
These cells then grew for five weeks in an incubator that mimicked the conditions inside the human body. According to the researchers, the results showed significantly improved recellularisation.
“It’s comparable to transplanting a ‘reconditioned’ liver, said Mayana Zatz, HUG-CELL’s principal investigator and co-author of the article. “It won't be rejected because it uses the patient’s own cells, and there’s no need to administer immunosuppressants.”
Extracellular matrix of a decellularised liver | Image Credit: HUG-CELL/USP
Obviously, there is a yawning gap between proof of concept and the operating theatre, but the goal is to scale up the process to create human-sized livers, lungs, hearts, and skin for transplant patients.
“The plan is to produce human livers in the laboratory to scale,” said lead author Luiz Carlos de Caires-Júnior to Agência FAPESP. “This will avoid having to wait a long time for a compatible donor and reduce the risk of rejection of the transplanted organ."
This technique could also be used to repair livers given by organ donors that are considered borderline or non-transplantable. “Many organs available for transplantation can’t actually be used because the donor has died in a traffic accident,” Caires-Júnior added. “The technique can be used to repair them, depending on their status.”
Even if we are at the early stages of this approach, it bodes well for future research. And for those on the organ transplant list, a reconditioned liver would be as good as a new one – complete with their very own factory settings.
Read the paper here: https://www.sciencedirect.com/science/article/abs/pii/S0928493120337814
Galen (129-216 CE) is one of the most famous and influential medical practitioners in history but he was also a scientist, an author, a philosopher, and a celebrity. He wrote hundreds of treatises, travelled and studied widely, was the physician to three emperors, and left a legacy of scientific thought that lasted for fifteen hundred years — even today, his work has an influence.
Header image Editorial credit: Eray Adiguzel / Shutterstock.com
He grew up in Pergamum, an intellectual centre of the Mediterranean world, in a wealthy family that encouraged him to pursue academia and funded his travels to learn in the best environments available, acquiring the latest techniques in medicine and healing.
He understood that diet, exercise, and hygiene were essential for good health and put that into practice in the four years he spent working for the High Priest of Pergamum's Gladiator School. This was a high profile and high pressure role and we know he reduced the death rate dramatically in his four years there. The recommendation he got helped secure him a position in Rome, capital of the empire.
He was not popular in the city — at one point, he seems to have been chased out by the local physicians, who strenuously disagreed with his methods — but he was eventually summoned by the emperor Marcus Aurelius to be his personal physician. He was described by the emperor as, “First among doctors and unique among philosophers".
Galen; Line engraving | Credit: Wellcome Images, Wikimedia Commons
Galen continued to navigate the difficult political environment of the imperial capital and was personal physician to two more emperors, while publishing prolifically and becoming one of the most well-known figures in the Roman Empire. Much of his work is lost to us but we still know a great deal about him, including that he had a flair for showmanship and controversy.
In the Greek world where he grew up, dissections had been common — of animals and humans. In Rome, this was not the case. In fact, human dissections were banned across the empire shortly before Galen arrived in the city. Undaunted, he gave a number of public anatomical demonstrations using pigs, monkeys, sheep, and goats to show his new city what they were missing (this was one of many incidents that contributed to local dislike of his methods as well as his increasing fame).
His legacy was huge, both because he recorded and critiqued the work of others in his field and because of the huge volumes of his own observations and theories. His texts were the foundation for much of medical education in the Islamic, Byzantine, and European worlds until the 17th Century.
The ban on human dissection likely limited his progress in some areas and many of his theories have (eventually) been disproved, such as the theory of the four humours — blood, black bile, yellow bile, and phlegm — based on Hippocrates' system and elaborated, as well as the efficacy of bloodletting.
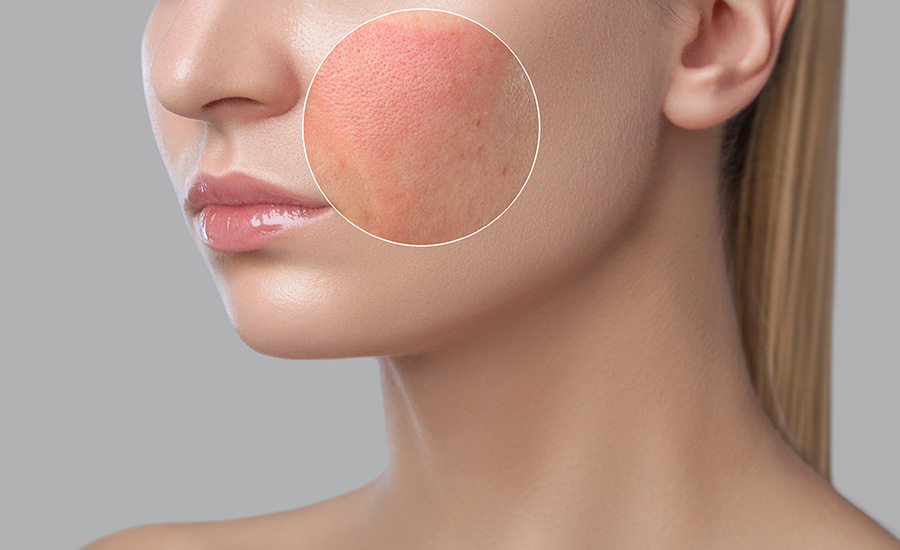
Galen observed that cataracts could be removed.
In other areas, however, he was remarkably successful. He observed that the heart has four valves that allow blood to flow in only one direction, that a patient's pulse or urine held clues to their disease, that urine forms in kidneys (previously thought to be the bladder), that arteries carry liquid blood (previously thought to be air), that cataracts could be removed from patients' eyes, among others. He also identified seven of the 12 cranial nerves, including the optic and acoustic nerves.
His focus on practical methods such as direct observation, dissection, and vivisection is obviously still relevant to modern medical research. Indeed, scientists who disproved his theories, such as Andreas Vesalius and Michael Servetus in the 16th century, did so using Galen's own methods.
The study of his work remains hugely important to the history and understanding of medicine and science, as well as the ancient world. The Galenic formulation, which deals with the principles of preparing and compounding medicines in order to optimise their absorption, is named after him.