BY DAVID BOTT
There is an old saying that “no strategy survives first contact with the enemy” (though I prefer Mike Tyson’s more descriptive version “everyone has a plan until they get punched in the mouth”!). When we were planning the Flue2Chem project, we drew out a detailed Gantt chart with deliverables, dependencies and deadlines. Sadly, the elapsed time between the last post in this series and now is a vivid example that Mike Tyson got it right.
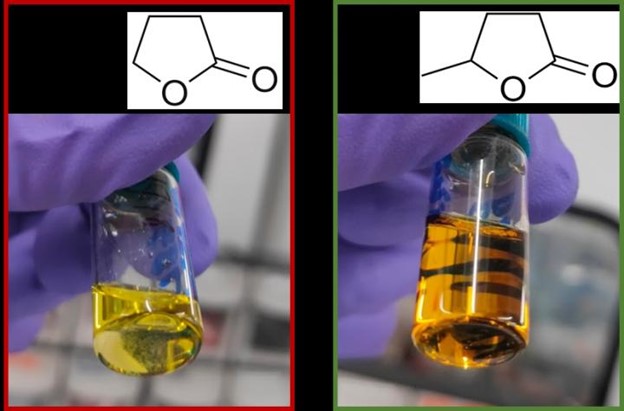
To recap, the aim of the Flue2Chem project is to collect the carbon dioxide from flue gases and turn it into a simple non-ionic surfactant for use in detergents and other consumer goods – potentially eliminating the need to extract more fossil fuel to make them. Key intermediates are dodecanol and ethylene oxide, but the first step is to capture the carbon dioxide.
What has gone before
The idea of extracting carbon dioxide from flue gases has been around for a long time, and has actually been “practiced” since the 1950’s. For a long time, it has been driven by the idea that carbon dioxide emissions can be captured and stored underground, thus avoiding adding them to the atmosphere and causing climate change. Nowadays, if you go to a Carbon Capture Utilisation and Storage (CCUS) conference, the few talks on utilisation mostly talk about the direct use of carbon dioxide either in fizzy drinks or for pumping into greenhouses as a feed to horticulture. We are aiming for something different.
The first need is for a source of flue gases. When we were putting together the Flue2Chem consortium we understood that different sources would have different chemical compositions, so we sought out different types of sources to maximise the spread of data for both the techno-economic and life cycle analyses. There are two paper companies in the consortium, Holmen and UPM. Their carbon dioxide emissions are classified as “biogenic”. Both have biomass combined heat and power plants, built in the days when biomass was regarded by the government as a renewable power source, and subsidised under the renewable obligations scheme that formed part of the 2009 Renewable Energy Directive. They each generate about 1000-1500 tonnes of carbon dioxide a day.
We also had the Port Talbot site of Tata Steel as part of the project. You could classify the carbon here as “used fossil carbon”. Coal is used both as a source of heat and as a reducing agent to turn the iron ore into iron. It is a complex process and so there are many sources of carbon dioxide on the Port Talbot site, some mixed with carbon monoxide In total, they generate about 15000-20000 tonnes of carbon dioxide a day.
The basic requirement for a process to capture carbon dioxide is easy to state – you need a system that will reversibly absorb carbon dioxide, and some good engineering!
The liquid amine route for capturing carbon dioxide uses a mixture of amines to react with the carbon dioxide to form carbonates. The absorption is usually carried out in a vertical column where the amine trickles down in a packed column and the carbon dioxide flows up. The resulting carbonate is then moved into another column where it is heated to decompose the carbonate to reform the amine and release the carbon dioxide. The energy efficiency of the process is largely determined by the energy required to decompose the carbonate. Over the years, different companies have optimised their mixture to minimise the energy costs and often keep this as “black art”.
Solid state absorption systems rely on physisorption. They used to be based on zeolites, but many recent ones use metal-organic frameworks. The early ones used a similar temperature driven process to control the absorption and desorption, but there are now systems based on pressure swing, where the absorption is driven by higher pressure and the desorption by much lower pressures. These are suggested to use lower energy than the more conventional temperature driven systems.
So, how is it going?
Early on in the project, one of the two companies providing the capture systems we wanted to include in the project – Carbon Clean – ran into an issue with the Environment Agency’s policy regarding solvent disclosure. They use an amine based solvent and, as mentioned above, they want to protect the confidentiality of their IP from this major commercial risk. However, the Environment Agency requires disclosure of any chemicals that might be emitted in any process, and most amines have a measurable partial pressure at the temperature used in the carbon capture process, so although they might have been able to get an exemption for a research or test use, once they go commercial in the UK with their system, they will have to disclose to the Environment Agency. AND, the Environment Agency is subject to Freedom of Information requests and would have to disclose Carbon Clean’s proprietary information. This is why Carbon Clean chose to withdraw its technology from the project, while continuing to provide techno-economic analysis.
This led to another decision – this time by Tata Steel. They had already worked with Carbon Clean in India and were looking to scale up the technology to the 10 tonnes/day envisaged within the project. With that off the table, they wanted to rethink their plans. As they were doing so, the bigger announcement that they would close the blast furnaces and move to use electric arc furnaces at Port Talbot amplified their concerns. Depending on the exact implementation route they choose, there might be minimal emission of carbon dioxide, so they withdrew from the work package to collect carbon dioxide.
Fortunately, in addition to Carbon Clean, we had also included a solid state capture technology, albeit at a much lower state of technology development, in the project. FluRefin had been developed at the University of Sheffield and was being commercialised by Carbon Capture and Utilisation International (CCUI). This had been operated at the small scale but as part of the project, it was being scaled up to 1 tonne/day capture. This required wholly new equipment, some of which had to be imported from India, some from Germany, but was assembled in the UK. It was planned to be installed at the first collection site (Holmen) at the end of November 2023, was actually delivered to site in mid-January, but commissioning issues delayed the first real carbon capture until late April. We have learnt a lot about fast-tracking process development – and the challenges it causes, partners working off different versions of the Process and Instrumentation Diagrams, the design experts being in Sheffield and the equipment being in Workington and so on but, as anyone who has done this before will tell you, this is all quite normal and we were very optimistic in our initial plans! Once on site in Workington, we had the support of some excellent engineers and the various problems were overcome.
One aspect of using a pressure swing process is the need to compress the input gas. This required the use of a number of compressors, but when they arrived we discovered that they had been designed for compressing air to be used as “compressed air” and were a bit “leaky” on the input side. We knew this because the output carbon dioxide concentration was lower that the input and not as we needed and thought we would get. This required more engineering to adapt the compressors for our use.
Another requirement of using the pressure based system is that the input flue gases need to be cooled (from about 150oC to around 30oC) – this recovers a fair amount of heat. More engineering was required!
Over the next few months, we started to capture enough carbon dioxide to supply the chemical conversion work packages. But this led to another “challenge”. We are aiming to capture about 1 tonne per day. This is below the level where we could engage one of the major gas product companies to provide bottling technology, and we were initially not planning to liquefy the gas (so did not have the required equipment). This means we were using a fairly basic “put gas in a pressurised gas bottle” process. Luckily, the University of Sheffield team were also involved in another UK Research & Innovation (UKRI) project – called SUSTAIN Steel. They had a small carbon dioxide liquefaction kit. We have “borrowed” it and are using it to liquefy the captured carbon dioxide, albeit at a very slow rate. We have other ideas for how to do this at a larger scale, but this works, and we are now capturing the required amount of carbon dioxide to send to the two centres where the next stage of our supply chain – the conversion of the carbon dioxide to ethylene oxide and dodecanol – will be carried out, but that’s another post!
So, what have we learnt?
Firstly, that project plans written in a hurry to fit within the proscribed timescale and budget will almost certainly be too optimistic and liable to require drastic adjustment. This was no real surprise to those who had been involved in scaling up processes before, but we rediscovered the saying that “if it can go wrong, it will” is irritatingly true. However, the capabilities of the individual organisations in the project and the creativity of the combined “leadership team” means that we have always found a way out of every “challenge”. We have also applied to Innovate UK to extend the project by 4 months, and have been successful, so have bought a little more time. The next work packages, which would have been squeezed by the 6 month (or so) delays will be “less” squeezed.
Perhaps the biggest learning is the need for flexible resources to enable scaling up the sort of processes we are using. This does not necessarily mean expensive new buildings or plants. A small carbon dioxide liquefaction plant that could handle 1-10 tonnes a day would have saved us about 2 months. More engineering expertise in the consortium might have saved us another couple of months building and commissioning the FluRefin plant. We had some allowance for creep in the original plan but when every month the deadline slipped by a month, I felt sorry for the project manager!
What has really been driven home to us is that the change we are attempting to prove – that it is feasible to move the chemistry supply chain away from virgin fossil carbon as a feedstock – may be scientifically credible, but reducing anything from theory to reality is harder than we think and required even more planning and effort than we imagined.
And we need to use realists, or even pessimists, as planners!!
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
Science is hard work. Understanding the world around us well enough to predict the behaviour of everything from sub-atomic particles to planets requires insight, patience, imagination and rigour. But science also lays the foundations of many of the industries that have changed our world – from pharmaceuticals to airplanes.
It is this application of science to address societal challenges that benefits people. And one of the biggest challenges is moving away from using virgin fossil carbon to feed the chemical supply chain!
It is worth stating at this point that, as we have talked about this work, we meet many people who do not understand how the chemistry using industries underpin other supply chains. We have had to explain many times to disbelieving audiences that cleaning products are currently mostly made from oil!
At the moment, the many branches of the chemistry using supply chains start with about 2.6 billion tonnes of carbon dioxide equivalent produced from oil and gas extracted from the fossil reserves (about 5-6% of the carbon extracted annually goes into this use). There are other sources of carbon and the science to use them is proven, but we need to start development and implementation. This means designing and building manufacturing capacity that operates efficiently to produce the volume of material needed to satisfy the current and future market needs – and this is a lot!
Technology, Development, Innovation – whatever you call the process of turning science into products is also hard, but in a different way. Something may be scientifically possible, but to make a product out of it for less than the price people will pay for it and at a volume that all the people that want it will be satisfied can be difficult. And competing with an established route where the feedstocks are cheap and easily available, and the processes have been optimised and scaled over decades, requires tenacity.
Flue2Chem is an Innovate UK funded consortium of 16 organisations that could make up a wholly new supply that produces the materials we currently use at a large scale but without starting from virgin fossil carbon. Rather than trying to do everything all at once, it is focused on a single product – a surfactant that is widely used in a range of cleaning products. It is one way to re-imagine the future of those parts of chemistry based industries that currently use virgin fossil carbon as a feedstock. The project is focused on demonstrating that carbon dioxide can be collected from flue gases and, by a series of chemical steps, turned into that common surfactant. Although it is focused on a single product, the processes and the learning from scaling them up could be applied more widely.
Flue2Chem consortium members
The challenge of scaling-up
Making a lot of anything usually involves making it in a large factory – and the more product needed to satisfy the market demand, the larger the factory. We are mainly talking about chemistry here, so the core manufacturing unit is a reactor. In the laboratory, most people think that chemistry is done in test tubes, but the truth is that the most common reaction vessel is probably a sub 1 litre round-bottomed flask. This is where the science of the basic reactions is tested and optimised. The next step is then a vessel between 5 and 25 litres in size. This is where we first encounter scaling laws! If we make a reaction vessel which is twice as big in the linear sense, it has 8 (23) times the volume and 4 (22) times the surface area. Most chemical reactions involve either the absorption or emission of heat – the amount of heat (absorbed or generated) increases with the volume of the reaction, but the heat must move through the walls of the vessel. So, a reactor three times as big (the 1 litre to 25 litre example above) generates or absorbs 27 times the heat but it must move through 9 times the surface area. The reactor surfaces must be three times as thermally conductive to allow this. The 25 litre flask is often exactly the same design and made of the same material as the 1 litre flask, so this does not happen!
Given that chemical reactors can have a capacity up to 1,000,000 litres these are substantial size differentials! Here the ratio between the initial reactor and the final, at-scale reactor means the challenges for this simplest reaction parameter must change 1000 times.
And thermal management is not the only challenge. Mixing also requires more energy at larger scales; removing the by-products of the reaction is also more problematic as reactor size increases.
But working at large scale has some commercial advantages – the cost of capital equipment needed usually scales at a lower rate than the science gets hard, and the complexity of the ancillary equipment can also be optimised, making larger factories more commercially attractive. Since big factories are often more commercially attractive, the technological challenges have been worked on for decades and large reactors for the currently used reactions are well optimised!
This optimisation, or scaling-up of the process nearly always goes in steps, and the size of those steps depends on the confidence of those in charge! These days, a lot of these steps can be carried out using computer based process software – decades of scaling things up has given industry experience of and insight into the critical factors that need to be considered, but moving to new reactions means the basic information must be measured again!
And this is just to get it to work!
But how big should you make it? An important factor in determining the size of the reactor is the size of the market – and therefore how much product you need to make.
So, how much do we need to make to prove this new supply chain will work?
For most people, the scale of the chemistry based industries are not even recognised, let alone considered.
At present the petrochemicals industry, which provide the bulk of feedstocks to the chemistry based industries globally uses about 2.6 billion tonnes of carbon dioxide equivalent a year. To give an idea of scale, if all that was made into polyethylene (commonly used in packaging, toys and cars) it would be a cube just under a kilometre along each length.
As already described, the target molecule of Flue2Chem is a simple surfactant with a chain of 12 carbon atoms as the oleophilic (the bit that attaches to the dirt) end and 5 to 7 ethoxy units as the hydrophilic (the bit that dissolves in the water) end. It is widely used for all sorts of cleaning products – globally figures of around 17,600,000 tonnes a year are quoted. This is equivalent to about 44,000,000 tonnes of carbon dioxide equivalent – so about 1.7% of the global petrochemicals market! What sounds a lot rapidly becomes small when you look at the actual numbers!
And how is it playing out for the Flue2Chem supply chain?
When we started the project, we had two carbon capture streams – an established liquid based system capable of collecting 10 tonnes/day and a start-up solid state system with a capacity of 1 tonne/day. Given that we also had three sites from which to capture carbon dioxide and the plan was to run each for 30 days, that would have given us an input of just under a 1000 tonnes of carbon dioxide.
At the other end of the supply chain, the final assembly of the surfactant could be carried out at about 1000 tonnes and the consumer products companies could use all that output to carry out test marketing of at least three products.
We rapidly discovered that the bottleneck would be the chemistry to turn carbon dioxide into ethylene oxide (for the hydrophilic end of the surfactant) and dodecanol (for the oleophilic end). The thermo-catalytic routes might be able to make about a kilogramme of the ethylene oxide and probably much less of the dodecanol. And on the biotechnology side of the options, although there were companies making ethanol (a precursor to ethylene oxide) from flue gases, they were not using carbon dioxide as the source. Biotechnology routes are often attractive because they make a single product, but using the biobased route to dodecanol looks like it would be hard to make even a kilogramme.
This would give us a few problems, but as the project progressed, we ran into another challenge. The company with the liquid system decided to withdraw from active carbon capture in the UK (the subject of a later blog), and one of the carbon sources got a government grant to radically overhaul their plant to drastically lessen the amount of carbon dioxide they might produce, so they have “paused” their direct involvement in that part of the project. We had now gone from a capacity of 990 tonnes to 60 tonnes. This was still more than enough to keep the chemists happy, but it gave us another opportunity to be innovative. The two carbon dioxide sources were in Cumbria and North Ayrshire, but the chemistry to convert it was being carried out in Sheffield and Germany and the biology in North Yorkshire. How could we transport that amount of carbon dioxide? At about 1000 tonnes, it might have been possible to “rent” a bottling facility and install it at the capture sites. At less than 100 tonnes, the equipment did not even exist! The solution we are adopting is to pressurise about 3 kilogrammes of carbon dioxide in pressure vessels and transport it from site to site. This limit on how much carbon dioxide we can transport from the emitters to the converters puts another size restriction in place, so a sizable fraction of the carbon dioxide we capture will have to go back into the flue! This is not ideal, but given the goal is to validate a wholly new, more sustainable supply chain and the lack of any other options, we don't have much choice.
There will be more…
Stepping stones to wisdom – what have we learned?
Perhaps we should have checked our numbers before we submitted the proposal, but since any scale-up project is designed to see how difficult it is to scale-up, discovering that there are issues beyond the technological is good learning.
The chemical and biological restrictions are a result of the lack for scale-up facilities in the UK, and we currently have no other options. If we are to develop the new chemistries required to switch feedstocks away from virgin fossil carbon to carbon dioxide, waste biomass or recycled plastics and oils, we will need several levels of scale beyond those currently available.
Underpinning this last point is the fact that many do not understand how the chemistry using industries underpin other supply chains – even their own. We have had to explain the range of products made from virgin fossil carbon (IEA figures are plastics packaging 36%, upholstery, carpets and paints 16%, textiles 15%, home and personal car products 10%, pharmaceutical and agricultural products 11%, car interiors and tyres 7% and electrical 4%) many times and often find ourselves trying to convince disbelieving audiences that cleaning products are currently made from oil! This means that many in government do not see the need to invest at the national scale in them in a coordinated manner. Scaling-up chemistry is generally not well catered for.
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
There is much talk of “decarbonisation” these days, and you could be forgiven for thinking this means eliminating carbon from all human activities. But there are lots of things that need carbon to exist. The chemistry of carbon is unique and nature relies on it for many things. Carbon has direct influence on conditions for life on our planet, whether or not you factor in the specific needs of human beings! And we depend on carbon chemistry for life too – our bodies burn carbon based fuels ourselves (grains, vegetables and meat are all carbon based materials).
We have also depended for millennia on carbon-based materials as clothing (skins, cotton and wool), housing (wood and thatch), and much more – until about 6000 BC when we started using inorganic materials (metals!) as well. With the birth of the oil age in the late 19th century, we started to make synthetic materials, initially striving to reproduce the properties of the natural materials we were used to – polyester was a cotton like material, polyamides were meant to be like silk, polyacrylonitrile was similar to wool in properties. In healthcare we started making naturally occurring therapies (like aspirin and morphine) at scale using synthetic chemistry. As we understood the relationships between chemical structure and properties during the 19th century, we moved on to more complex chemical structures. We cannot get away from carbon because so many planetary systems depend on it, so getting it rid of it altogether is not really an option!
So, what do we mean when we say “decarbonisation”?
The problem is not with carbon dioxide as such, but with the amount we have put into the ecosphere over the last 150 years or so years and the effect it is having on the climate.
In the past there was a lot more carbon than we currently have in the atmosphere – somewhere between 15-30 times as much – but then life wasn’t the same in the Mesozoic period – evidence suggests the average global temperature was 10-15 degrees higher, so species like us would probably not have survived. Over the intervening 150,000,000 years, a variety of natural processes absorbed and sequestered that carbon in rocks, coal, oil and gas. But coal, oil and gas are an easily available source of energy, and their extraction has powered progress over the last 200 years! And when we burn them, the carbon that was previously safety locked up in the ground goes back into the atmosphere.
So what is the problem?
The amount of carbon dioxide we have generated over the last 150 years can be found in the atmosphere, the oceans and on land. This is the product of the coal, oil and gas we have extracted and burnt over the same time period. The problem is that the Earth’s climate cannot cope with this increase, and we need to stop.
Here is how it all adds up:
Atmosphere – Since the beginning of the industrial revolution, the amount of carbon dioxide in the atmosphere has increased from around 2.2 to 3.3 trillion tonnes – meaning we have added about 1.1 trillion tonnes.
Oceans – There is a lot of focus on carbon dioxide in the atmosphere and the impact it has on climate, but carbon dioxide is also absorbed by the oceans, where it also has an impact – the water becomes more acidic and damages marine ecosystems. For every tonne of carbon dioxide in the atmosphere there is about 0.75 of a tonne in the oceans. This means there is probably about 800 billion tonnes in the oceans.
Land – There is also work that indicates that about 8.3 billion tonnes of plastic waste have been produced since 1950 – roughly equivalent to 26 billion tonnes of carbon dioxide. Since plastics account for about 30% of the petrochemical products stream, we can work out that 87 billion tonnes of carbon dioxide from the use of oil and gas as a feedstock is also in the ecosphere.
Total – This means we can estimate that we have put about 1.99 trillion tonnes of carbon dioxide into the ecosphere since we started using coal, oil and gas as a source of energy.
The amount that we find in the ecosphere is almost identical to the amount extracted from the geosphere over this time period. Evidence that this is a man-made effect can be seen by comparing this number to the 2.2 trillion tonnes of carbon dioxide equivalent that industry records show the coal, oil and gas industries have extracted from the planet over the last 150 years. The small difference is probably due to the accuracy of the figures, which have been collected over 150 years!
We often fall into the trap of differentiating between fossil carbon and biogenic carbon, as if biogenic carbon is acceptable, but we have been putting fossil carbon into the atmosphere for so long that it makes up about 35% of the carbon in the atmosphere and has therefore been absorbed by plants and will make up roughly the same fraction of what we call biomass! This means that 35% of the biogenic carbon is really second generation fossil carbon. This becomes important when one source is favoured over another by regulations.
The goal is not to stop using carbon – it is to stop taking it out of the ground and putting it into the ecosphere!
If the various government promises to achieve Net Zero are kept and we do manage to stop using fossil carbon as a fuel by 2050, where will we get the carbon we use as a chemical feedstock that we have come to rely on as the source of materials such as plastics, fertilisers, packaging, clothing, digital devices, medical equipment, detergents or tyres? Currently we use about 2.6 billion tonnes of carbon dioxide equivalent a year to make a wide variety of things – through a “petrochemical” supply chain. We cannot “decarbonise” that without inventing new materials to deliver all of the products, but we can “defossilise” it – which means we stop using virgin fossil carbon as its feedstock.
Where will the carbon come from then?
There are three sources of carbon (biomass, recycled plastics/oils/solvents and carbon capture and utilisation) widely touted as replacements for all that fossil carbon. All are essentially recycling previously used carbon. They tend to get framed as competitors for the role, but if you analyse the amounts available, the likely costs of using them, and the timescales needed to get them to the right scale it looks like we will need them all!
Back to Nature
Biomass refers to using biological sources for the raw materials – wood, crops, animals and bacteria are good examples. Advocates rightly point out that many synthetic materials are clones of natural materials. They tend to forget that society moved to synthetic analogues because there wasn’t enough to satisfy societies needs in terms of volume and price, but global supply chains were less sophisticated back then.
However, from the generally accepted numbers, there would be enough raw materials – the planet produces about 50 billion tonnes of biomass a year – of which about 14 tonnes is produced by man. Of that, it is estimated that about 1.8 billion tonnes of “waste” is produced a year – mainly cellulosic. This produced in farms and food processing plants. Of the biomass that goes to food, we waste about 30-40%, so can add another 1.6 billion tonnes, but this is produced in restaurants and houses, so collection might be expensive! And finally, we have the waste we humans and our animals directly produce. Estimates vary but are usually about 500 million tonnes of human waste and almost 3 billion tonnes from farm animals! There would be enough carbon in biomass to satisfy the requirements, but the logistics of collecting it might be a challenge (and probably involve the generation of more carbon dioxide – there is always a trade-off where transport is involved). Also, biological processes can often be slow and less atom efficient than the currently used chemical processes, but this can be accommodated by appropriate systems design.
Nevertheless, the use of biomass as a feedstock for chemicals is already here and growing in scale.
Second time around
All the carbon that currently goes out in the form of products could form the basis of a different recycling route – often called “chemcycling”. Here the previously used plastics, oils and solvents can be partially broken down into reactive chemical species like those used in the manufacture of the original products. This is usually done with heat, and so costs energy. It also often uses solvents or extra chemistry. Given the range of materials that will make up the input, there will probably need to be a sorting process at the front end. And, like the waste biomass, the input materials will be collected from geographically distributed sites, so will need to be transported to plants where the recycling will take place (with the potential carbon dioxide emissions as a result).
Mining the problem
Finally, we could use the largest source of carbon already in the ecosphere – the carbon dioxide we have been putting into the atmosphere. With its current concentration of about 0.05%, it is a daunting task. However, we can “practice” how to make it work with a higher concentration (often 10-15%) – when we burn things, we produce a gas stream with a higher concentration of carbon dioxide and send it up a chimney or flue. We will probably go on burning things for a few years yet and using them as a source of carbon will not only moderate the amount we put into the atmosphere in the short term, but also enable us to refine the technology so that we can one day we can remove carbon dioxide from the atmosphere and reverse the damage we have done to the climate – although sadly it would take hundreds of years to do so.
Are we there yet?
All three routes to providing a non-virgin fossil source of carbon are being worked on already. However, they are all at such a small scale it is difficult to judge their efficiencies and effectiveness.
- Biomass is probably the most technologically advanced, but scaling-up the processes and addressing the collection and logistics issues are important challenges that need to be overcome.
- Recycling the “second generation” carbon cannot supply enough – it is limited by the efficiencies and costs of all the processes from collection to chemical recycling but can probably contribute a sizable fraction of the requirement.
- Carbon Capture and Utilisation (CCU) is the least well advanced but with a roadmap to Direct Air Capture (DAC) (the slightly different name for capturing the carbon dioxide direct from the atmosphere) offers the most compelling argument for investment – if DAC can be made to work commercially, it can be used to remediate the atmosphere. Net zero targets aim to take us back to 1990 levels of carbon dioxide in the atmosphere by eliminating the use of fossil fuels. Depending on the scale and speed at which DAC can be implemented, it might be possible to get back to pre-Industrial Revolution atmospheric carbon dioxide levels in several hundred years!
Carbon Capture (CC) has rather had its reputation tarnished by the pervasive suggestion that it can be used to capture (and store) carbon dioxide so effectively we can go on using fossil fuels for many years. Most analysts outside the oil and gas industry do not see how the maths works!
All of the routes are years away from making a major contribution to replacing the volume of carbon based feedstocks used to make the wide variety of products (and the market is growing at over 5% per year, which means that it will double in size every 12 or so years). We need to start soon and grow quickly to make any meaningful impact.
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
One of the questions we often get asked is ‘how did you assemble such a large project so quickly?’ The short answer is ‘we didn’t, it took years!’, but the story is important
In the last blog we discussed the scale of the use of petrochemicals to make things society doesn't know are made of fossil carbon, and the drive to change this – this time we will look at how we built the team.
On page 17 of the Chemistry Council Strategy published in 2019, you will find the phrase ‘Sustainable Materials for Consumer Products’. This is (we think) the first time the phrase was used in this context,. and so was effectively the beginning of the story.
Technically, there is prehistory. The UK government had tried twice before to understand and support the “chemistry-using” industries but had failed to capture the breadth and scale of their impact on the economy in their engagement. They had included the pharmaceutical companies alongside the more traditional “chemicals” companies, but not the extensive downstream activities that used chemicals to deliver a product or process. In 2018 they had spread their net a bit wider and included the consumer companies who use chemistry to make a large number of products for a whole range of markets. These companies were seemingly more aware of the way consumers were looking at the discussion on climate change and who were wondering why the products they could not live without had to be made from fossil carbon and contribute to climate change. So, these companies started making commitments to be fossil carbon free by 2030 or not long after. The scale of their production (often millions of tonnes) meant that the necessary changes to their supply chains had to be at a similar scale and they quickly found that their suppliers (and their suppliers) could not easily accommodate the changes they wanted to see in their chosen timeframe. What was required was a more radical rebuilding of their supply chains.
The Society of the Chemical Industry (SCI), who were responsible for the Innovation Committee of the Chemistry Council, decided to build a community who could do something about this challenge.
The first workshop in the area was held at SCI on 22 February 2020. It included participants from retailers (Marks & Spencer, Sainsbury’s, Walgreens Boots Alliance and Enkos), consumer products manufacturers (Unilever, GSK, Reckitt Benckiser and Proctor & Gamble) a number and variety of chemical manufacturers (Croda, Innospec, Johnson Matthey, Thomas Swan, BASF, INEOS, Lubrizol and Synthomer), universities/RTOs (CPI, University of Bath and University of York) and several companies engaged in the recycling of materials (Biffa, Symphony Environmental).
The discussion was outcome-oriented, and we finished the day with a draft list of things to work on in the second workshop. But within a few weeks COVID happened and we went into lockdown! As they learned to cope with Zoom and Teams, the core team met online and developed the ideas from the workshop. First, the tasks were sorted into three main buckets – engagement, new chemistries and measurement.
Talking to people
Under the engagement banner, we recognised the importance of explaining what we understood were the challenges and how best to address them. Audiences would include chemistry-based companies in the supply chain, regulators and policy makers in government, and consumers. Initial attempts at engagement rapidly taught us that before we could explain what we were doing, we often had to make the audience aware of the ubiquity of carbon-based chemistry in everyday life and the relationship to climate change. One example was when I found myself being interviewed by Rachel Johnson on LBC about the impact of laundry on climate change and her not believing that the cleaning products themselves were made from fossil carbon resources!
The idea of starting with awareness raising, moving on to (gentle) discussion of the science and technology before even mentioning the “ask” became our approach!
Changing the way we do things
The new chemistries banner was an area where we were all more comfortable. If we were to avoid using fossil carbon as a feedstock, we needed another source of carbon, or we needed to find the functionality the businesses needed using another element. The second option looked very difficult – and therefore expensive. The options for the first were well known – “biomass” (ill-defined but very popular as a choice), recycled (second generation?) plastics and oils (logistics is the current issue) and carbon dioxide (lots of it about, but more energy needed to get it to a useable state).
Then we agreed (being mostly from business) that we needed a specific target at focus on.
If we could start with a non-fossil source of carbon and turn it into a useful molecule, we would have demonstrated that there was a completely different way of making things. In the process we could learn what was possible, what impact this alternative would have on the environment, whether it was commercially viable and what social impact it might have.
Since there are so many things made of fossil carbon – packaging, paints, adhesives, textiles, carpets, upholstery, tyres, drugs, fertilisers, insulation and cleaning products – selection was difficult, but the team knew a lot about cleaning products, so we started there. After a lot of swapping of anonymised data, we alighted on non-ionic surfactants. They are extensively used in consumer cleaning products (about 1,000,000 tonnes a year) and they are relatively simple molecules. These are made up of a hydrophobic end (usually a 12-14 carbon atom chain) and a hydrophilic end (usually made up of 5-7 ethylene oxide units).
Alongside the search for a way to make this sort of chemical without using fossil carbon, we realised that cleaning products were almost invariably flushed down the drain – and that often that meant straight into streams and rivers. We knew from other studies that they would degrade into other chemicals, and eventually into carbon dioxide – but we could not find any studies that mapped the complexity of that degradation pathway – was it a biological process, or was it photolytic, or a mixture of both? We talked to BBSRC. We talked to NERC. To date we have not been able to interest any UK funding agency in researching this aspect of what happens to these carbon based molecules in the environment, although there is lots or research into the effect of the chemicals on the environment!
Measuring what was going on
The third area we pondered over was how to measure what was going on – both currently and as a result of anything we might do. We looked at various life cycle analysis studies and quickly realised there were real differences between academic studies – and even consultancy or in-house techniques. Given that we knew we would be building a wholly new supply chain, how were we going to measure the impact of our actions?
Getting on with it
We knew better what we had to do. The next problem was to find a way of funding the work. It took almost a year of talking to the KTN and Innovate UK before we became aware of the Transforming Foundation Industries competition. In the late Summer of 2021, we decided to develop a proposal to turn carbon dioxide from flue gases into our target surfactant.
The specification of the competition meant that we had to work with other foundation industries. Given that we wanted a source of carbon dioxide, and they mostly produce it, this gave us options. We built links to paper companies such as Holmen and UPM. They often use biomass boilers as heat and energy sources. These are currently classed as zero emissions under the emissions trading scheme, but the rules are tightening and are looking for the next technology to remain viable. We also talked to Tata Steel, who use coke both as a fuel and reducing agent in their blast furnaces. We tried to talk to cement manufacturers but were unsuccessful.
Next, we needed a means to capture the carbon dioxide in their flue gases. There are basically 3 ways to capture carbon dioxide, solvent adsorption, solid phase adsorption and membranes. We contacted Carbon Clean (who use solvents) and were already talking to the University of Sheffield (who were developing a solid phase process). Both were interested and so joined the consortium.
Turning carbon dioxide into the C12 fatty alcohol (to make the hydrophobic end of the surfactant) and the ethylene oxide (to make the hydrophilic end of the surfactant) was the next goal. The BASF, University of Sheffield and Johnson Matthey all had expertise in thermo-chemical processes which could achieve this and all were interested. We knew there would be biochemical processes that could achieve the same goal but no companies in the UK, so we turned to the Centre for Process Innovation (part of the High Value Manufacturing Catapult) and found they were working in the area so added them to the consortium.
Croda were already the supplier of choice to react these two components together to make the final surfactant.
We then had the consumer product companies, Unilever, Proctor & Gamble and Reckitt, who would evaluate the surfactant and “prove” that getting the carbon from a different source did not change the properties.
We also folded in our concerns about measuring the impact of what we had done by including the UKRI Interdisciplinary Centre for Circular Chemical Economy to evaluate the life cycle and technico-economic aspects of the various processes we were evaluating so that we could put together a justified case for a new supply chain at the end of project.
The Confederation of Paper Industries also joined to be able to understand what we had done and recommend it to other paper companies.
And the SCI joined to help with exploitation and dissemination as the project progressed.
Are we there yet?
We submitted our Expression of Interest to Innovate UK just before Christmas 2021 and (mostly) didn't worry about it until the New Year. On 10 January, we learned that our application was successful, so buckled down to writing the full application.
The consortium submitted its proposal on 6 April 2022
As we understand it there were eight proposals submitted but only money for four, but we were still disappointed that the initial funding announcement did not include us! However, somehow Innovate UK found some more money and the remaining projects were judged to be fundable so on 14 July we learned that we had the money!
For those who haven’t applied for Innovate UK funding before, the detailed process is simple but rigorous. One really, really important step is for every company to sign a “Collaboration Agreement”. This sets out the goals of the project, how it will be managed, and how the intellectual property that arises during the project will be allocated. It is often a source of contention, and the amount and intensity of the contention seems to scale geometrically with the number of partners in the consortium. Given that many of the partners were more used to competing than collaborating and they all had lawyers, it seemed at times to scale exponentially! However, because the scientific leads in all the companies had worked together (some, at this point, for over 3 years) and there was real commitment to get started, (after a small hiccup) we all signed the agreement just before Christmas 2022. It was then that we discovered we all had to sign the Grant Offer Letter as well, but we managed that too.
Then the work started…(see Part 3)
The exam question
So, how did we assemble the consortium?
- It took time, and lots of interaction. The core of the consortium – Unilever, P&G, Reckitt, Croda, BASF, Johnson Matthey and the SCI – had all been sharing ideas, challenges, opportunities and frustrations for over 3 years. Individually some had also worked with the others we invited to join, so there were always personal recommendations and shared experiences.
- It took transparency and honesty. The relationships we have built over those years are strong. We have disagreed (a fair amount) on the way, but we have always shared – and even strengthened through our interaction – a commitment to move from fossil carbon feedstocks to a sustainable chemistry based industry.
- It required shared commitment, an understanding of how the different organisations fitted into what will be a wholly new supply chain, and tireless advocacy both within our own organisations – and to anyone who will listen outside them!
- It needed the patience of a saint!
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
A collaboration of 15 organisations is part way through an Innovate UK funded project to turn the carbon dioxide in flue gases into non-ionic surfactants for cleaning products. Many have asked: why we are doing it? how did we build the team? and how things are going? This is the first part of that story.
No one really thinks about the role of chemistry in society. Although everything around us is chemistry – life itself, the natural world, and the material world we have created to add to the natural world – we mostly talk about “chemicals” in a derogatory way. Does society understand how much it relies on carbon-based chemicals? Does chemistry need a PR agency?
Why we need carbon
It is difficult to say exactly when the “synthetic” world started, but the huge expansion came with the use of fractions of the oil and gas we were extracting from the ground as a fuel as material feedstocks about 120 years ago. The petrochemicals supply chain now accounts for about 2.6 billion tonnes of carbon dioxide equivalent a year (about 5.7% of the carbon extracted) and is growing at about 8% a year – therefore roughly doubling every 10 years. And the products at the end of the many supply chains that start with these fossil feedstocks range from things we cannot do without (such as disinfectants, soaps, textiles for clothes and so on) to things we “like” to have but would probably fight to keep (such as cosmetics and flying)!
The focus is often on single-use plastic packaging, which makes up about 30% of that 2.6 billion tonnes. But there are many other products that depend on the same source – products for use in the home (carpets, upholstery, paint and adhesives) at about 16%, textiles (mostly for clothes) at about 15%, pharmaceuticals, agrochemicals and fertilisers at about 11%, cleaning products and cosmetics at about 10%, the interiors of cars and their tyres at about 7% and the use in electrical and electronic products (both insulation and cases) at about 4%. Such is society’s use of these products, that we cannot simply stop using them, or make them from something other than carbon. We will need a source of carbon at the same scale.
At this point in the narrative, it is worth pointing out that although many uses of fossil carbon as a fuel can be replaced by alternative technologies, aviation fuel is different. Although there are prototype electric planes for short haul flights, and visions of hydrogen powered planes at some point, the options for long haul air travel look distinctly limited – with really only Sustainable Aviation Fuel as a runner. The aviation industry would need about 1.3 billion tonnes of carbon dioxide equivalent a year to keep going at their current rate, so about half that needed to supply our material needs. It faces the same challenge as the carbon based materials – if they do not use fossil carbon, where would it come from?
Where we can get carbon from
The source that gets talked about a lot is “biomass”. At the global level, it is estimated that about 100 million tonnes of biomass is produced each year, roughly half on the land and half in the sea. This equates to roughly 200 million tonnes of carbon dioxide equivalent. Most reviews discount the marine biomass as difficult to harvest and then calculate that of the land based only about 8 million tonnes (so, 16 million of carbon dioxide equivalent) a year can be harvested “sustainably”.
Another factor often quoted is the logistical cost of harvesting, since for some crops the energy required to harvest compromises the efficiency of the process. The factor that doesn’t get talked about much is the difference between the biological building blocks and the petrochemical ones we are used to – which would require us to build a different set of supply chains and result in products with different properties.
The second source is the materials made of fossil carbon we have already extracted. There are various estimates of how much plastic is in landfills, but the average seems to be about 5 billion tonnes of plastic – about 15 billion tonnes of carbon dioxide equivalent. This would need to be chemically recycled back to feedstocks equivalent to those that underpin the current supply chains.
The third source is the carbon dioxide we have been “storing” in the atmosphere. Since the start of the industrial revolution when our emission of carbon dioxide started in earnest, we have emitted close to 2.2 trillion tonnes of carbon dioxide – half of which is in the atmosphere and the rest has partitioned into the oceans. The problem is that it is very dilute, so you would have to capture a lot of “air” to get meaningful amounts of carbon dioxide – but people are trying!
However, since we are still burning a lot of fossil fuels (and biomass) at the moment, we have flues and chimneys where the carbon dioxide concentration is many times higher. Why not start there and increase the process efficiency with experience?
However, there is a challenge with using carbon dioxide as a source for carbon-based materials. Carbon dioxide is where carbon tends to end up in an oxygen-rich environment – particularly where there is light. It is basically at the bottom of a thermodynamic well and it needs a lot of energy – and green hydrogen – to get it back to those feedstocks we need to go on making things that we want in a way that keep the cost down.
What next?
So, we have a very large problem, some options – each of which has advantages and “issues”, and it will take a long time to change our supply chains, so we need to act fairly quickly before our filling the atmosphere with carbon dioxide does irreparable damage to our environment. We need a plan…
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
Creating a paper pulp bottle that holds different liquids was a challenge that led BASF to join forces with Pulpex. Using sustainable chemistry the partners came up with an award-winning formula.
-
Vikki Callaghan, Packaging Project Manager, BASF plc
-
Tony Heslop, Senior Sustainability Manager, BASF plc
-
Scott Winston, CEO Pulpex Ltd
Could you start by explaining how the collaboration and the idea for the product came about?
Vikki: We had an existing relationship with Diageo. BASF and The Innovation Team at Diageo had worked on other projects addressing packaging needs. When the team had this idea for an innovative packaging solution they came to us. The challenge put to us was ‘Do you have the chemistry that will hold many different liquids in a paper pulp bottle?’ I love a challenge and was excited to get talking.
Scott: Having worked with BASF before, they were our natural choice to explore this conundrum. Diageo had the idea and an early proof-of-concept of a paper bottle, but it wasn’t utilising sustainable chemistry. The intellectual property was in place but the transformation of scientific proof-of-principle to scaled commercialised technology wasn’t something that could be done alone. The partnership with BASF naturally continued into Pulpex as it formed and continued to grow, remarkably, throughout the Covid-19 lockdown. BASF’s corporate purpose to create chemistry for a sustainable future was intrinsically aligned to meet our need to deliver a commercialisable product that could be produced at scale.
Tony: Following that first call in November 2019, we got together a couple of weeks later and enjoyed an intense deep dive workshop. This was going to take some time but if successful we knew this could be an impactful innovation. We set to work!

Testing out their bottle in the lab. Image courtesy of Pulpex Ltd.
What hurdles did you overcome in the development of the material?
Tony: The obvious hurdle was the pandemic. There were two years between our first and second face-to-face meeting. My initial thought was how do you drive an innovation process when you can’t get together. Surely constructive and productive collaboration isn’t possible? In fact, the inability to travel meant that we could talk more frequently despite our different geographical locations. Once we’d set up weekly online meetings, which evolved into smaller specialist break out groups, the process actually had many positives and the relationships, as well as the innovation, flourished.
Vikki: Of course, as with any innovation, we experienced technical challenges, too. There was no overall solution because we were looking at very diverse requirements and specifications. Different brand owners with different liquids meant there were many considerations and customised solutions required.
Sustainable packaging is a growing market with new products being launched. Can you explain where your product fits in and how is it different from similar materials?
Scott: Pulpex recognises the need to balance three critical aspects. Firstly, new packaging must continue to deliver established brand equity and meet consumer expectations on quality; secondly, any new packaging must technically deliver on performance through the supply chain starting with filling infrastructure compatibility and through distribution and critically, at end-of-life the packaging must be recyclable in existing infrastructure from collection to enable circularity, or where it does unfortunately escape to the environment, it must degrade and not leave an unintended legacy.
Vikki: The resulting fibre bottle is lightweight and offers brand owners a sustainable, environmentally-friendly alternative to plastic and glass bottles.

The final product. Image courtesy of Pulpex Ltd.
What are the main markets for the packaging? Are you able to comment on customers already using your product?
Tony: The innovation will be aimed at brand owners who want to have an alternative sustainable type of packaging, a product that is suitable for ‘on the go’ and that is easily recyclable through existing waste streams. The technology will hold a range of liquids from alcohol and detergent to shower gel, ketchup and engine oil.
Scott: Trials of the finished product have already started to take place with the most recent being at a corporate five-a-side football tournament at Wrexham AFC in May, where several hundred bottles were put to the test working with Severn Dee Water. Branded especially for the event and designed as a keepsake, the feedback from the public was resoundingly positive and it was great to see our bottles in action supporting those on the pitch.
What are the next steps for the BASF/Pulpex collaboration?
Scott: Having developed such a sustainable alternative packaging, our continuing sprint is scaling up! The technology has been developed and we are expecting to have bottles on shelves soon.
Vikki: We will have our ongoing quest of looking to hold a vast range of liquids and for different brand owners. We will have customised solutions, in different sizes, different shapes… the innovation and collaboration continues.

BASF and Pulpex won the SCI Innovation Enabled by Partnership Award 2023. Image credit: Andrew Lunn Photography
Links to previously published articles and videos (BASF/Pulpex/SCI)
- BASF May 2023 – SCI Award
- BASF August 2021 – Collaboration with Pulpex
- SCI News May 2023 – SCI Award
- Pulpex May 2023 – SCI Award
- Pulpex video
Rarely have science and government been as clearly linked as the initial response to the Covid-19 pandemic, when politicians could be heard claiming they were being ‘led by the science’ as often as they could be seen doing that pointing-with-a-thumb-and-fist thing.
This Thursday, the UK’s Chief Scientific Adviser, Sir Patrick Vallance, will receive the Lister Medal for his leadership during the Covid-19 pandemic, and you can stream it live here, exclusively on SCI’s YouTube channel!
In readiness for Sir Patrick’s lecture, Eoin Redahan looks back at three ways science helped to mitigate the spread of Covid-19.
People will never look at vaccine development the same way. For good or ill, we have realised just how quickly they can now be developed. Similarly, we have realised what can be achieved when the brightest brains come together. These are two of the positive legacies from Covid.
But there are others. Some of the innovations conceived to tackle Covid will now tackle other pathogens. Here are just three of the innovations that emerged…
1. Wastewater warning

As Oscar Wilde once said: ‘We are all in the gutter, but some of us are looking up at the genetic material in stool samples.’
Not many people would find inspiration in wastewater treatment plants when thinking about early warning systems for infectious diseases.
Nevertheless, during the Covid-19 pandemic, researchers at TU Darmstadt in Germany came up with a system that detected Covid infection rates in the general population by analysing their waste – a system so accurate they could detect the presence of Covid among those without recognisable symptoms.
To do this, they examined the genetic material in samples from Frankfurt’s wastewater plants and tested them using the PCR test. They claim that their measurement was so sensitive it could detect fewer than 10 confirmed Covid-19 cases per 100,000 people.
It is inevitable that Covid-19 variants will rise again, but this system could alert us to the need for tighter protective measures as soon as the virus appears in our wastewater.
2. UV air treatment
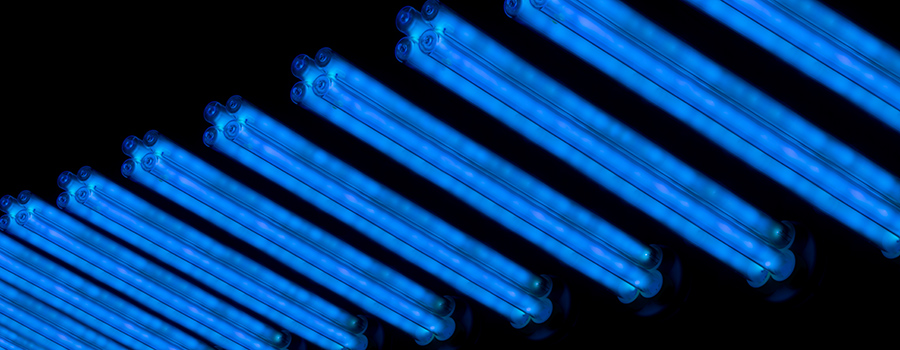
UV light can reportedly reduce indoor airborne microbes by 98%.
Warning systems are important, as are ways to stop the spread of pathogens. To do this, a team from the UK and US shed light on the problem – well, they used ultraviolet light to remove the pathogens.
Using funding from the UK Health Security Agency, Columbia University researchers discovered that far-UVC light from lights installed in the ceiling almost eliminate the indoor transmission of airborne diseases such as Covid-19 and influenza.
The researchers claim it took less than five minutes for their germicidal UV light to reduce indoor airborne microbe levels by more than 98% – and it does the job as long as the light remains switched on.
‘Far-UVC rapidly reduces the amount of active microbes in the indoor air to almost zero, making indoor air essentially as safe as outdoor air,’ said study co-author David Brenner, director of the Center for Radiological Research at Columbia University Vagelos College of Physicians and Surgeons. ‘Using this technology in locations where people gather together indoors could prevent the next potential pandemic.’
3. Biological masks?

‘Physical mask, meet biological mask.’
Many moons ago, it was strange to see a person wearing a mask, even in cities with dubious air quality. Now, they are ubiquitous, and it would appear that mask innovations are everywhere too.
During Covid, researchers from the University of Granada in Spain were aware that wearing masks for a long time could be bad for our health. They devised a near field communication tag for inside our FFP2 masks to monitor CO2 rebreathing. This batteryless, opto-chemical sensor communicates with the wearer’s phone, telling them when CO2 levels are too high.
In the same spirit, researchers in Helsinki, Finland, developed a ‘biological mask’ to counteract Covid-19. The University of Helsinki researchers developed a nasal spray with molecule (TRiSb92) that deactivates the coronavirus spike protein and provides short-term protection against the virus – a sort of biological mask, albeit without those annoying elastics digging into our ears.
‘In animal models, nasally administered TriSb92 offered protection against infection in an exposure situation where all unprotected mice were infected,’ said Anna Mäkelä, postdoctoral researcher and study co-author.
‘Targeting this inhibitory effect of the TriSb92 molecule to a site of the coronavirus spike protein common to all variants of the virus makes it possible to effectively inhibit the ability of all known variants.’
The idea is for this nasal spray to complement vaccines, though during peak Covid paranoia, it might be tricky persuading everyone on the bus that you’re wearing a biological mask.
Covid disrupted scientific progress for many, but as we know, invention shines through in troublesome times. Plenty of innovations such as the ones above will make us better equipped to tackle air borne diseases – alongside the stewardship of leaders like Sir Patrick Vallance.
Watch Sir Patrick Vallance’s talk – Government, Science and Industry: from Covid to Climate – at 18:25 on 24 November
What does clean smell like? What if the fragrance you want to create is that of a sweet-smelling, yet poisonous, flower? In his Scientific Artistry of Fragrances SCITalk, Dr Ellwood led us by the nose.
When Dr Simon Ellwood spoke about creating a fragrance, it sounded like a musical composition or a painting. The flavourist, sitting before a palette of 1,500 fragrance ingredients. Each occupies a different note on the register: the top notes, the middle ones, and the bottom.
To the outsider, this seems impossibly vast and daunting. The Head of Health & Wellbeing Centre of Excellence – Fragrance and Active Beauty Division at Givaudan mentioned that Persil resolved to come up with ‘the smell of clean’ for its detergents in the late 1950s.
But what should clean smell like? Should it be the green, citrusy aromas of this laundry detergent, the smell of mint, or the antiseptic at the hospital?
To make choosing smells slightly less daunting for flavourists and perfumers, they are at least split into odour families such as citrus, floral, green, fruity, spicy, musky, and woody. Some of these ingredients are natural, some are inspired by nature, and others come from petrochemicals and synthetic materials.

The delicious-smelling musk deer.
Deer gland perfume
One of the smells you may have sprayed on your person – one sibling in this odour family – has peculiar origins. The pleasant, powdery smell known as musk was originally extracted from the caudal gland of the male musk deer and from the civet cat.
But as the Colognoisseur website notes, as many as 50 musk deer would have to be killed to obtain one kilogramme of these nodules. Now, killing a load of deer and cats for a few bottles of perfume may not have seemed unethical several centuries ago, but it also wasn’t sustainable or cost-effective. It became clear that a synthetic musk was needed.
When the synthetic musk discovery came in 1888, it was a chance discovery. Albert Bauer had been looking to make explosives when a distinctive smell came instead, along with the scent of opportunity.
>> Read about the science behind your cosmetics

Dior recreated the woodland notes of Lily of the valley.
Do you like the smell of jasmine?
Dr Ellwood’s talk laid bare not only the vastness of everything we smell, but also the ingenuity of those who recreate these odours. In terms of breadth of smell, neroli oil – which is taken from the blossom of a bitter orange – has floral, citrus, fresh, and sweet odours, with notes of mint and caraway. Similarly, and yet dissimilarly, jasmine’s odour families are broken down into sweet, floral, fresh, and fruity, and – jarringly – intensely fecal.
The ingenuity of flavourists is exemplified by lily of the valley. The woodland, bell-shaped flowers are known for their evocative smell, but all parts of the plant are poisonous. Despite this, French company Dior synthetically recreated the lily of the valley smell in its Diorissimo perfume in 1956 using hydroxycitronellal, which is described by the Good Scents Company as having ‘a sweet floral odour with citrus and melon undertones’.

Cyanide smells like almonds, but you might not want to eat it.
Of course, as Dr Ellwood noted, synthetic flavours can only ever get so close to the real thing – an imperfect facsimile. However, the mere fact that chemists have recreated deer musk, lily of the valley, and the prized ambergris from sperm whales to create the fragrances we love is almost as extraordinary as the smells themselves.
‘Fragrance,’ he said, ‘will always be the confluence of the artistry of the perfumer and the chemist.
Register for our free upcoming SCI Talk on the Chemistry behind Beauty & Personal Care Products.
Little machines that blend makeup tailored for your skin alone… Technology that details the tiny creatures walking on your face… The cosmetic revolution is coming, and Dr Barbara Brockway told us all about it.
Max Huber burnt his face. The lab experiment left him scarred, and he couldn’t find a way to heal it. So, he turned to the sea. Inspired by the regenerative powers of seaweed, he conducted experiment after experiment – 6,000 in all – until he created his miracle broth in 1965. You might know this moisturiser as Crème de la Mer.
A rocket scientist in the world of cosmetics seems strange, but when you interrogate it, it isn’t strange at all. As Dr Barbara Brockway, a scientific advisor in cosmetics and personal care, explained in our latest SCItalk, cosmetics hang from the many branches of science.
Engineering, computer science, maths, biology, chemistry, statistics, artificial intelligence, and bioinformatics are among the disciplines that create the creams you knead into your face, the sprays that stun your hair in place. They say it takes a village to raise a child, and it takes an army of scientists to formulate all the creams, gels, lotions, body milks, and sprays in your cupboard.

Some say sea kelp can be used to treat everything from diabetes, cardiovascular diseases, and cancer, to repairing your nails and skin.
There is a reason why the chemistry behind these products is so advanced. If you sell bread, it is made to last a week. If you make a moisturising cream, it is formulated to last three years. To make sure it does that, chemists test it at elevated temperatures to speed up the time frame. They conduct vibration tests and freeze-thaw tests to measure its stability.
Dr Brockway likened the process of bringing a product to market to a game of snakes and ladders. If you climb enough ladders, you could take your own miracle brew to market within 10 months.
But expectations are high, and the product must delight the user. Think of the teenager who empties a half a can of Lynx Africa into his armpit, or the perfume that is a dream inhaled. Each smell she likened to a musical composition.
But these formulators are not struggling artists. Perfumers and cosmetic chemists – these bottlers of love and longing and loss – can earn a fortune. Dr Brockway’s quick calculation provided a glimpse of the lucre.
Take 15kg of the bulk cream you mixed on your kitchen table. That same cream could be turned into 1,000 15ml bottles, each sold for £78. So, just 15kg of product could fetch £78,000. So, it’s easy to see why the global beauty market is worth $483 billion (£427 billion), with the UK market alone worth £7.8 billion – more than the furniture industry.
Smart mirror, mirror, on the wall…
It’s unsurprising that an industry of such value and scientific breadth embraces the latest technologies, from those found in our phones to advances in genetics and the omics revolution.
Already, the digital world has left the makeup tester behind. Smart mirrors overlay virtual makeup, recommend products for your complexion, and even detect skin conditions. Small machines that look like coffee-makes blend bespoke makeup. Indeed, Dr Brockway noted that Yves Saint Laurent has created a blender that produces up to 15,000 different shades.
Even blockchain has elbowed into the act. It is being used to make sure that a product’s ingredients aren’t changed in between batches. By showing customers every time-stamped link of the supply chain, companies can prove that their products are organic or ethically sourced. The reason why blockchain is significant here is that, once recorded, the data stored cannot be amended.
At first glance, proving the provenance of materials to customers might seem like a marketing ploy, but this is also being done in response to the increasing fussiness of the consumer.
Collagen is the main component of connective tissue.
Dr Brockway said all brands are now under pressure to incorporate sustainability into their business practices. The younger age group is also looking for more organic, vegan-friendly ingredients, and businesses have had to respond.
For example, microbial fermentation is being used instead of roosters’ coxcombs to create hyaluronic acid. Similarly, Geltor claims to have created the first ever biodesigned vegan human collagen for skincare (HumaColl21®). Such collagen is usually provided by our friends the fish.
These advances are significant, certainly to the life expectancies of roosters and fish, but of such ingredients revolutions are not made. Other forces will shake the industry.
Meditating on omics
Back in the 1970s, scientists thought the microbes that live on our skin were simple, but next-generation DNA technology reveals that thousands of species of bacteria live on our skin (a pleasant thought). Dr Brockway says these microbes tell us about our lifestyles – to the point that they even know if you own a pet.
So, what is the significance of this? Developments in DNA technology and omics (various disciplines in biology including genomics, proteomics, metagenomics, and metabolomics) mean we can now get not just a snapshot, but an entire picture of what’s going on on your face.
‘Thanks to omics we really know what’s now going on with our skin and see what our products are doing,’ Dr Brockway said. ‘We know the target better. We know which collagens, out of the 263, we need to encourage.’
We are learning more and more about how our skin behaves. And those time-honoured potions and lotions espoused by our grandparents – it will make sense soon, not just why they work, but why they work for some and not for others. In cosmetics, we are leaving the era of checkers and entering the age of chess.
This is the first of three cosmetic SCItalks between now and Christmas. Register now for the Scientific artistry of fragrances.
In his winning essay in SCI Scotland’s Postgraduate Researcher competition, Alexander Triccas, postgraduate chemistry researcher at the University of Edinburgh, explains how the tiny shells produced by marine algae protect our natural environment.
Each year, SCI’s Scotland Regional Group runs the Scotland Postgraduate Researcher Competition to celebrate the work of research students working in scientific research in Scottish universities.
This year, four students produced outstanding essays. In the fourth of this year’s winning essays, Alexander Triccas explained how coccoliths provide a valuable carbon store and could play a key role in keeping our bones healthy.
Why tiny shells produced by marine algae are important for both global carbon stores and repairing bones
Although humans can engineer complex and eye-catching structures that help us navigate through our daily lives, they are nowhere close to the design and functionality of natural materials.
These mineral structures are specifically grown to provide support, protection, or food for many organisms. Humans would not exist without them. Indeed, our bones and teeth are made of calcium phosphate. But when grown in a lab, calcium phosphate forms as simple rectangular crystals, which is vastly different to how our bones and teeth look.
This is because our bodies use organic molecules to precisely control how minerals grow, producing materials that can fulfil very specific tasks. Biominerals can even be produced inside single cells. Coral reefs are held together by calcium carbonate minerals made by marine invertebrates. Elsewhere in the ocean, carbonate shells produced by small algae cells are buried on the ocean floor, over time forming the chalk rocks that make up coastal landmarks such as the White Cliffs of Dover.

Advances in microscopy are shedding new light on the composition of coccoliths.
This process is incredibly important to the environment. It takes carbon dissolved in seawater, turns it into solid material, then stores it at the bottom of the ocean. It is concerning then that we don’t know how ocean acidification and rising CO2 levels will affect coccoliths, the name given to these carbonate shells.
>> SCI’s Scotland Group connects scientists working in industry and academia throughout Scotland. Join today!
We’re still unsure how coccoliths are produced, particularly how organic molecules are used to give them their unique shape. Proteins and sugars decide where and when the first carbonate mineral forms; then the growth of the coccolith is controlled by sugar molecules.
But how exactly do these organic molecules control the mineral that is produced? We struggle to answer this question because we don’t know how the composition of the coccolith changes as the structure grows.
Composition of the coccolith
Our research focuses on imaging coccoliths in an attempt to observe these changes. We used a technique called X-ray ptychography to map coccolith composition over the course of its formation. This revealed that coccoliths are not entirely made of calcium carbonate, instead having a hybrid structure containing mineral and organic molecules. But this isn’t all.
We revealed that the composition of the coccolith changes during its growth. We think this could represent a transition from a disordered liquid-like state to an ordered crystalline state. While this is common in other biomineral-produced organisms like corals, no evidence of this transition has been reported in coccolith formation before.
>> Read Rebecca Stevens’ winning essay on PROTAC synthesis.
This is incredibly important because it tells us how the cell is controlling the first calcium carbonate mineral that forms. The transition enables the cell to control exactly how it wants the mineral to form, meaning coccoliths can be made faster.
It might also lessen the impact that more acidic seawater has on mineral formation. This could mean coccoliths will not be affected by ocean acidification as much as expected, which is good for the planet’s long-term carbon stores.
However, this is only a prediction. Improvements to the microscopes used to analyse coccoliths will help us know if the transition occurs. Electron and X-ray microscopes are extremely useful in industry – from drug research and medical imaging, to data storage and materials analysis – but their use in these fields is still relatively novel.
Coccolith analysis could give us a better idea of how bones are produced.
Most advancements in instrumental procedures are done in academic research. Our work, therefore, helps us understand the benefits and limits microscopes may have, making them more suitable for industrial use.
Bone research also relies heavily on these microscopes. Our findings could be important in understanding how bones are produced, benefiting not only pharmaceutical and medical industries, but also improving human healthcare by providing better treatments to patients.
In her winning essay in SCI Scotland’s Postgraduate Researcher competition, Marina Economidou, first year PhD Student at GSK/The University of Strathclyde, talks about palladium recovery.
Each year, SCI’s Scotland Regional Group runs the Scotland Postgraduate Researcher Competition to celebrate the work of research students working in scientific research in Scottish universities.
This year, four students produced outstanding essays in which they describe their research projects and the need for them. In the second of this year’s winning essays, Marina Economidou explains the need for palladium recovery and making it more efficient.
Pictured above: Marina Economidou
U-Pd-ating the workflows for metal removal in industrial processes
Palladium-catalysed reactions have great utility in the pharmaceutical industry as they offer an easy way to access important functional motifs in molecules through the formation of carbon-carbon or carbon-hetero-atom bonds.
The superior performance of such reactions over classical methodologies is evident in modern drug syntheses, where Buchwald-Hartwig, Negishi or Suzuki cross-coupling reactions are frequently employed.
However, the demand for efficient methods of palladium recovery runs parallel to the increased use of catalysts in synthesis. The interest in metal extraction can be attributed to several reasons.
Cross-coupling steps are usually situated late in the synthetic route, resulting in metal residues in the final product. In addition to possessing intrinsic toxicity, elemental impurities can have an unfavourable impact on downstream chemistry.Hence, their limit must be below the threshold set by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
The need for palladium recovery
However, the importance of palladium recovery does not only arise from the need to meet regulatory criteria. The volatility of palladium supply as a result of geopolitical instabilities has been a focus of attention this year, with Russia producing up to 30% of the global supply and prices reaching an all-time high of £81,179 per kilogramme.
Therefore, aside from the need to remove metals from the product for regulatory reasons, there is a desire to recover metals from waste streams as effectively as possible due to their finite nature and high costs.
The sustainability benefits of recovery for circular use are an additional incentive for an efficient extraction process, as catalysts can be regenerated when metal is returned to suppliers.
The increasing pressure for greener processes and more ambitious sustainability goals – such as GlaxoSmithKline’s environmental sustainability target of net zero impact on climate by 2030 – also contribute to the need for further refinement of working practices.
>> SCI's Scotland Group connects scientists working in industry and academia throughout Scotland.
Palladium has many uses including in catalytic converters, surgical instruments, and dental fillings.
Improving extraction processes
It is essential to have well-controlled and reproducible processes for pharmaceutical production, as redevelopment requires further laboratory work and additional time and resources.
With several industry reports on the inconsistent removal of palladium following catalytic synthetic steps, there seems to be a knowledge gap as to which factors affect the efficiency of extraction and why there can be significant differences between laboratory and plant conditions.
The focus of my PhD is investigating the speciation of palladium in solution in the presence of pharmaceutically relevant molecules, to offer an insight into the efficiency of metal extraction at the end of processes.
By understanding the oxidation state and coordinative saturation of the palladium species formed in the presence of different ligands, a better relationship could be established between the observed performance of metal extraction processes under inert and non-inert conditions.
With the wide breadth of ligands and extractants that are now commercially available for cross-coupling reactions, my ambition is to generate a workflow for smart condition selection that not only achieves selective metal recovery, but is scalable and can be transferred to plant with consistent performance.
The cost and preciousness of metal catalysts are both factors that prohibit their one-time use in processes. Understanding how palladium can be extracted and recovered in an efficient manner will not only deliver reliable processes that meet the demands of the market in the production of goods, it will also lead to economic and environmental benefits.
>> Read Angus McLuskie’s winning essay on replacing toxic feedstocks.
>> Our Careers for Chemistry Postdocs series explores the different career paths taken by chemistry graduates.
The Commonwealth Games has landed in Birmingham. Before the Games began, viewers were treated to an extraordinary opening ceremony (featuring a giant mechanical bull) and its artistic director, Iqbal Khan, was lauded for his ingenuity.
But such ingenuity shouldn’t surprise any of us, for Birmingham has long been a place of outsized invention. For more than 300 years, the inhabitants of this industrial powerhouse have churned out invention after invention; and its great pragmatists have turned patents into products.
Chemistry, too, owes a debt to the UK’s second city. Whether it’s the first synthesis of vitamin C, the invention of human-made plastic, adventures in mass spectrometry, or electroplated gold and silver trinkets, Birmingham has left a lasting legacy.
Here are five chemists whose innovations may have made an appearance in your life.
Alexander Parkes – man of plastic

Plaque commemorating Alexander Parkes in Birmingham, England. Image by Oosoom
Look around you. Look at the computer screen, the mouse button you click, and the wire casings everywhere. Someone started it all. That man was Alexander Parkes, inventor of the first human-made plastic.
The son of a brass lock manufacturer from Suffolk Street, Birmingham, Parkes created 66 patents in his lifetime including a process for electroplating delicate works of art. However, none were as influential as the 1856 patent for Parkesine – the world’s first thermoplastic.
Parkes’ celluloid was based on nitrocellulose that had been treated by different solvents. In 1866, he set up the Parkesine Company at Hackney Wick, London, but it floundered due to high cost and quality issues. The spoils of his genius would be enjoyed by the rest of us instead.
Sir Norman Haworth – the vitamin seer

Sir Norman Haworth
Sir Norman Haworth may have been born in Chorley, Lancashire, but his finest work arguably came after he became Director of the Department of Chemistry in the University of Birmingham in 1925. Haworth is famous for his groundbreaking carbohydrate investigations and for being the first to synthesise vitamin C.
By 1928, Haworth had confirmed the structures of maltose, cellobiose, lactose, and the glucoside ring structure of normal sugars, among other structures. Apparently, his method for determining the chain length in methylated polysaccharides also helped confirm the basic features of starch, cellulose, and glycogen molecules.
However, Haworth is most famous for determining the structure of vitamin C and for becoming the first to synthesise it in 1932. The synthesis of what he called ascorbic acid made the commercial production of vitamin C far cheaper – the benefits of which have been felt by millions of us.
For his achievements in carbohydrates and vitamin C, Haworth received the Nobel Prize for Chemistry in 1937 (shared with Paul Karrer). He was the first British organic chemist from the UK to receive this honour. Haworth even had a link to SCI, having been a pupil of William Henry Perkin Junior in the University of Manchester’s Chemistry Department.
Francis William Aston – adventures in mass spectrometry

Blue plaque for Francis William Aston. Image from Tony Hisgett
Another Nobel Prize-winning chemist from Birmingham is Francis William Aston. The Harborne native won the 1922 prize for discovering isotopes in many non-radioactive elements (using his mass spectrograph) and for enunciating the whole number rule.
For a time, academia almost lost Aston, as he spent three years working as a chemist for a brewery. Thankfully, he returned to academic life and obtained concrete evidence for the existence of two isotopes of the inert gas neon before the first World War.
After working for the Royal Aircraft Establishment during the Great War (1914-18), he resumed his studies. The invention of the mass spectrograph proved pivotal to his discoveries thereafter. Using this apparatus, he identified 212 naturally occurring isotopes.
George Elkington and John Wright – all that glitters

George Elkington patented the electroplating process developed by John Wright. Image from Spudgun67
It isn’t surprising that George Elkington should become an SCI favourite, as he blended both scientific ingenuity with business. The son of a spectacle manufacturer patented the first commercial electroplating process invented by Brummie surgeon John Wright in 1840.
Wright discovered that a solution of silver in potassium cyanide was useful for electroplating metals. Elkington and his cousin Henry purchased and patented Wright’s process before using it to improve gold and silver plating.
The Elkingtons opened an electroplating works in the city’s now famous Jewellery Quarter where they electroplated cutlery and jewellery. And they didn’t do too badly out of it. By 1880, the company employed 1,000 people in seven factories.
Alfred Bird – winging it

1906 advertisement for Birds Custard powder. Image from janwillemsen
In 1837, Alfred Bird was in a pickle. He wanted to serve his dinner party guests custard, but his wife was allergic to eggs and yeast, and egg was the main thickening agent of this delicious gloop.
Instead of serving something else, the chemist shop owner invented his own egg-free custard by substituting cornflour for eggs. His guests found it delicious and Bird’s Custard was born.
Not content with this innovation, Bird is also credited with being the father of modern baking powder. Once again, his wife’s allergies were said to be the inspiration, as he wanted to create a yeast-free bread for her. In bread and custard, true love always finds a way.
By rethinking the way our products are designed and changing the way we use plastics, we can tackle the blight of marine litter and the general accumulation of plastic waste. But, as Professor Richard Thompson said in our latest SCItalk, systemic issues and historical excesses have made this no easy task.
Contrary to popular perception, plastic is not the villain. When it comes to marine littering, we are the ogres, with our single-use bottles bobbing in the oceans and the detritus of our everyday lives littering the coastline.
We are the reason why 700 species are known to encounter plastic debris in the environment. It is because of us that plastics have beaten us to the bottom of the deepest oceans and glint in the sun near the summit of Mt. Everest.
According to Richard Thompson, of the Marine Institute School of Biological and Marine Sciences at the University of Plymouth: ‘Plastic debris is everywhere. Its quantity in the ocean is likely to triple between 2015 and 2025.’
As Professor Thompson pointed out all of these facts to his audience in our latest SCItalk on 23 March, he outlined potential solutions. However, there is no ignoring the depth of the issues at hand when it comes to the litter in our seas.
The problems
1 - The weight of history
Society has gradually woken up to the menace of discarded plastics and, laterally, to the threat of microplastics and nanoplastics. The problem is that we left the barn door open decades ago. So, all of those plastic microbeads from shower gels, fibres from clothing, and tyre wear particles polluted our seas for many years before it came to public and scientific attention.
Professor Thompson said that 300 papers were published globally on microplastics in the last academic year alone, but research in the area was relatively thin on the ground before Thompson and his colleagues released their pioneering study on microplastics in Science in 2004.
2 - Bad habits
‘The business model for the use of plastics hasn’t really changed since the 1950s,’ Professor Thompson said. According to him, we have had 60 years of behavioural training to just throw products away, and our waterways reflect this attitude.
According to Professor Thompson, 50% of shoreline litter items recorded during the 2010s originated from single-use applications. Without a sea change in our attitude towards single-use items, this problem will persist.
>> Why are we ignoring climate change and what can we do about it? Read more on our blog post.
Microplastics have been subject to great scrutiny, but much of the research is quite recent.
3 - We need to talk about nanoplastics
The problems with larger plastics and even microplastics are now well documented. The worrying thing, according to Thompson, is that there are knowledge gaps when it comes to nanoplastics in the natural environment. What are the effects of nanoplastic ingestion? What are the effects of human health? Time will tell, but Thompson was keen to ask if we really need that information before we take action.
He was more sanguine about the effects of microplastics. ‘The concentration of microplastics is probably not yet causing widespread ecological harm,’ he said, ‘but if we don’t take measures, we’ll pass into widespread ecological harm within the next 50-100 years.’
The solutions
It seems counterintuitive to think of petrochemical plastics as a sustainable solution; and yet, despite the environmental problems posed by their durability, they do have a role to play in a greener approach.
‘If used responsibly, plastics can reduce our footprint on the planet,’ Thompson noted. Indeed, the lightweight plastic parts in our cars and in aviation can actually help reduce carbon emissions. But despite their merits, how do we keep plastic litter from our seas?
1 - Design for end of life… and a new one
To illustrate a flaw in the way we design plastic products, Professor Thompson gave the example of an orange coloured drinks bottle. While the bright colour may help sell juice drinks, there is an issue with recycling these coloured plastics because their value as a recyclate is lower. Clear plastics, on the other hand, are much more viable to recycle.
He argues that many products aren’t being designed with the whole lifecycle in mind. ‘We’re still failing to get to grips with linking design to end of life,’ he said, before highlighting the importance of communicating how products should be disposed of right from the design stage.
Basically, our products should be designed with end of life in mind. ‘If we haven’t even designed a plastic bottle properly,’ he lamented, ‘what hope do we have with something that’s more complicated?’
Those brightly coloured plastic bottles look nice and fancy, but they can be challenging to recycle in a circular economy.
2 - Ever recycle? Ever fail? Recycle again. Recycle better
Professor Thompson argued that better practices are needed to help divert materials away from our seas (and it should be noted that there are other types of discarded materials to be found there). If we recycle greater quantities of end of life plastic products and bring them into a circular economy, he said, ‘we’d decouple ourselves from oil and gas as the carbon source for new production because the carbon source we use would be the plastic waste’.
He said more could also be done with labelling so that customers know whether, for example, a product is compostable and which waste stream it needs to be placed in to achieve that. He also noted that addressing our single-use culture would be a good place to start if we want to change the business model of linear use.
3 - Broaden the discussion and pull those policy levers
The good news is that there is an appetite for change. ‘Ten years or so ago there was no consensus that there was a problem,’ Thompson noted. ‘I would argue that this has changed.’ However, he also feels that it is essential to gather reliable, independent evidence to inform interventions, rather than espousing solutions that could make things worse.
‘We need to gather that evidence from different disciplines,’ he said. ‘We need to have at the table product designers and couple them with the waste managers. We need to have economists at the table. We also need to bring in social scientists to look at behaviour. We’ve got to think about this in the round.’
He also felt that policy measures – such as mandating recycled content – could be a good option, along with better design and disposal.
The tools we need to tackle plastic pollution are already at our disposal. We just need to act more responsibly – which, unfortunately, has been part of the problem all along.
As Professor Thompson said: ‘It’s not the plastics per se that are the problem – it’s the way we’ve chosen to use them.’
>> For more interesting SCI talks like Professor Thompson’s, check out our YouTube channel.
>> Find out more about the work of Professor Thompson and his colleagues here: https://www.plymouth.ac.uk/research/marine-litter.
At COP26, Nikita Patel co-hosted the Next-Gen debate, where an inspiring group of young people discussed how chemistry is tackling climate change. The PhD student at Queen Mary University of London shares her experience.
While the United Nations Climate Change Conference (COP26) may be over, there is still plenty to be done in the fight against climate change. We’ve seen what can be achieved when we work together and no doubt science will play a key role.
On Thursday 4 November, I had the privilege of co-hosting the Countdown to Planet Zero Next-Gen debate organised by SCI to showcase the work being carried out by our young and innovative scientists to tackle climate change. It was a real pleasure to share the stage and hear from some great scientists, exploring the themes Fuels of the Future, Turning Waste into Gold and Engineering Nature. The event gave the audience the opportunity to question and challenge the panel members on their climate change solutions.
Panel L-R: Dominic Smith, Natasha Boulding, Clare Rodseth, Jake Coole, Nikita Patel, Oliver Ring (Brett Parkinson joined virtually).
While I was feeling nervous about my hosting duties, I was very excited at the same time as I knew how important it was to educate the audience, whether they were members of the public or aspiring scientists, on how science is crucial in battling the climate emergency.
An important part of my role as a host was to ensure the incoming questions and comments were understood by all, given the mixed audience attending. This highlighted how essential good science communication is to prevent misunderstandings and the spread of misinformation.
It was brilliant to see how engaged the audience were from the flurry of questions that came in during the session, so much so that we didn’t manage to get through all of them! There were a wide variety of questions aimed at particular panellists but also towards the panel as a whole. It was thought-provoking to hear how scientists from different backgrounds offered their own perspectives on the same topic.
4 November was also Energy Day at COP26 and the atmosphere was buzzing! I learnt a lot from attending the Green Zone, not only from our panellists but from all the exhibitors present too. I appreciate the small, individual actions we can each take that will make a difference but also the need to work together to achieve the common goal of fighting climate change. It was clear to see how science and business go hand in hand to provide solutions to society and how interdisciplinary collaboration is key.
The result of our poll question: ‘Do you think that science is pivotal in providing climate change solutions?’ spoke for itself, with a resounding yes from 100% of the audience participants! This was a very positive outcome and showed that it is not all doom and gloom when it comes to discussing the climate crisis.
On a personal level, I'm going to continue implementing some simple changes like using public transport more, eating more vegan food and flying less and aim to keep the discussion going with my peers as the climate emergency is far from over.
SCI team, panellists and hosts.
I hope the youth panel event has inspired the next generation of scientists and showcased some of the exciting work that is going on behind the scenes which people may not realise and ultimately, that there is hope in science.
>> To rewatch the event, the recording is available on the COP26 YouTube channel: Countdown to Planet Zero Combating climate change with chemistry | #COP26, and on our Climate Change Solutions hub.
>> Want to read more about the technologies discussed by our panel? Read our event review: https://www.soci.org/blog/2021/11/2021-11-05-cop26-review.
A group of inspiring young scientists took centre stage at COP26 on 4 November to show how the next generation of chemists is finding tangible climate change solutions.
In a day dominated by what countries pledged to stop doing at COP26, such as pursuing coal power and financing fossil fuel projects overseas, it was refreshing to learn about low-carbon technologies and the young people driving their development. At the Next Gen forum, we heard from an array of young chemists, all associated with SCI, who are at the sharp edge of this change.
We heard from Brett Parkinson, Senior Engineer of Low Carbon Fuels and Energy Technologist at C-Zero, who is working on commercialising a way to decarbonise natural gas. The California-based company’s technology converts the natural gas into hydrogen and solid carbon to provide a clean energy source while sequestering the carbon; and the aim is to have this process up and running next year.
Natasha Boulding is building towards Net Zero a different way – with a greener concrete. The CEO and Co-founder of Sphera has developed a lightweight carbon negative additive using waste plastics that aren’t currently being recycled. She says the company’s blocks are the same strength and price as existing concrete blocks, but with 30% more thermal insulation. There is also the added benefit of reusing waste materials that would otherwise have gone to landfill or been incinerated.
Another solution discussed by Dominic Smith, Process Development Engineer at GSK, reduces energy consumption through green chemistry. He is trying to find greener ways to make medicines using enzymes. These enzymes, which can be found in plants and soil, replace chemical synthesis steps to cut energy consumption during processing and reduce hazardous waste.
Panel (left to right): Dominic Smith, Natasha Boulding, Clare Rodseth, Jake Coole, Nikita Patel, and Oliver Ring (Brett Parkinson spoke via video link).
It was apparent from the discussion that many solutions will be needed for us to reach our climate change targets. On the one hand, Jake Coole, Senior Chemist in Johnson Matthey’s Fuel Cells team, is working on membrane electrode assembly for hydrogen fuel cells to help us transition to hydrogen-powered buses and trucks.
At the same time, Clare Rodseth, an Environmental Sustainability Scientist at Unilever, has been using lifecycle assessments to reduce the environmental impact of some of the 400 Unilever brands people use all over the world every day. For example, this work has helped the company move away from petrochemical ingredients in its home care products. ‘Even small changes,’ she said, ‘have the potential to bring about large-scale change.’
Incremental change
However, for each of the technologies discussed, barriers remain. For Coole and co., having a readily available supply of hydrogen and charging infrastructure will be key. And for Dominic Smith and his colleagues, the use of enzymes in green chemistry is still in its infancy; and getting enzymes that are fast enough, stable enough, and produce the right yield is difficult. Nevertheless, he noted that manufacturers are now using enzymes to produce the drug amoxicillin, reducing the carbon footprint by about 25%
And some things will take time to change. Natasha Boulding noted that concrete is the second most used material in the world after drinking water, and we simply can’t create many green technologies, such as wind turbines, without concrete foundations.
She said the construction industry is quite traditional but also pointed to perceptible change, with the green concrete market growing and companies becoming increasingly aware of their carbon footprints.
Collaboration was seen as crucial in producing climate change solutions.
The reality is that global action on climate change is recent. As Brett Parkinson said: ‘the main reason we’re talking about it now is that there’s a driver to do it. Until the last decade, the world hadn’t cared about CO2 emissions. They just talked about caring about it.’
How pivotal is science in all of this?
So, what could be done to make climate action more effective? For Parkinson, effective policy is key. He argued that if the market isn’t led by policies that encourage low-carbon innovations, then it won’t work as needed. ‘It all starts with effective decarbonisation policy,’ he said. ‘Legacy industries are very resistant to change. If you don’t have strong and consistent policies… then they’re not going to adapt.’
Another key to our low-carbon evolution is collaboration, and the SCI provides a confluence point for those in industry and academia to work together to produce innovative, low-carbon products. As Clare Rodseth said: ‘Collaboration is really important – linking up people who can actually come together and address these problems.’
As the discussion came to a close, you had the impression that the debate could have gone on for much longer. ‘Hopefully, we’ve demonstrated that there is action, and it’s being driven by young people like our panellists today,’ summarised Oliver Ring, the event’s co-Chair, before asking for the result of the audience poll.
The question: How many of those watching believed that science is pivotal in providing climate change solutions?
The answer: Just the 100%.
>> Thank you to Johnson Matthey for sponsoring the event, to the speakers for sharing their time and expertise, and to co-chairs Nikita Patel and Oliver Ring for doing such an excellent job.
SCI’s America International group has awarded the 2021 Perkin Medal to Dr Jane Frommer. The 114th Perkin Medal was presented to Jane at the Bellevue Hotel in Philadelphia, Pennsylvania, in recognition of her outstanding contribution to chemistry.
Dr Jane Frommer
Dr Frommer is renowned for her key contributions in electronically conducting polymers and scanning probe instrumentation. Her pioneering work with scanning probes paved the way for their use in chemistry, materials science and, eventually, in nanotechnology. According to SCI America, her nanoscopic analytic methods are vital to nanostructural research and are used across many industries.
Dr Frommer began her career in 1980 at Allied Corporate Laboratories (now Honeywell), where she created the solution state of electronically conducting organic polymers. In 1986, she joined IBM where, along with other instrumentalists, she demonstrated the ability to image and manipulate single molecules using scanning tunnelling microscopy. During her multi-year assignment at the University of Basel Physics Institute in the early 1990s, Dr Frommer’s team expanded the capability of scanning probes in measuring the functional properties of organic thin films with atomic force microscopy.
Since 2018, she has worked as a science advisor for Google. In this capacity, she has sought to increase the amount of open source data available in the physical and life sciences. She also helps Silicon Valley start-ups navigate the chemical and material challenges of nanotechnology and has mentored countless students and young scientists in high school, college, and in her laboratory in recent decades.
Previous recipients of the Perkin medal include Barbara Haviland Minor, of the Chemours Company, and Ann E Weber, of Kallyope Inc.
Dr Frommer has written more than 100 referred publications and is the co-inventor of more than 50 issued patents. With her extraordinary body of work spanning more than 40 years, she is a worthy recipient of the prestigious Perkin Medal.
The Perkin Medal is widely acknowledged as the highest honour in American industrial chemistry. It was established to commemorate the 50th anniversary of William Henry Perkin’s discovery of mauveine at the age of just 18. Perkin’s creation of mauveine, the world’s first synthetic aniline dye, revolutionised chemistry and opened up new frontiers in textiles, clothing, and other industries. Perkin was a founding member of SCI and this Medal was first presented to him in New York in 1906.
For more information on the Perkin Medal and the nomination process, visit: soci.org/awards/medals/perkin-medal
The War on Plastic is a grand title. To most of us, it doesn’t seem like much of a war at all – more like a series of skirmishes. Nevertheless, if you look closely, you’ll see that a lot of companies are tackling the issue.
GSK Consumer Healthcare (GSKCH) is one such organisation. The healthcare brand that gave us Sensodyne and Advil has launched a carbon neutral toothbrush to reduce our reliance on fossil fuels (which create virgin plastic).
The composition of its Dr. Best tooth scrubber is interesting. The handle comprises a mixture of a cellulose derived from pine, spruce, and birch trees and tall oil, which comes from the wood pulping industry. The bristles are made from castor oil and the plastic-free packaging includes a cellulose window.
According to GSKCH, Dr. Best is Germany’s favourite toothbrush brand and there are plans to apply the technology to toothbrushes across its portfolio, including its Sensodyne brand. At the moment, GSK needs to apply carbon offsetting initiatives to make the toothbrush carbon neutral, but it says it is working on future solutions that do not require this approach.
Net zero shopping
GSK isn’t the only company that is actively reducing the use of plastics and minimising waste. Supermarket chain Morrisons has made aggressive moves in recent years to cut waste, and has just launched six ‘net zero waste’ stores in Edinburgh that will operate with zero waste by 2025.
Customers at these stores will be able to bring back hard-to-recycle plastics such as food wrappers, foils, yoghurt tubs, mixed material crisp tubes, coffee tubs, batteries, and plant pots. At the same time, all store waste will be collected by a range of specialist waste partners for recycling within the UK, and unsold food will be offered to customers at a cheaper price on the Too Good to Go app.
Morrisons’ proactive approach will help find a new life for hard-to-recycle packaging.
‘We’re not going to reach our ambitious targets through incremental improvements alone,’ said Jamie Winter, Sustainability Procurement Director at Morrisons. ‘Sometimes you need to take giant steps and we believe that waste is one of those areas. We believe that we can, at a stroke, enable these trial stores to move from recycling around 27% of their general waste to over 84% and with a clear line of sight to 100%.
‘We all need to see waste as a resource to be repurposed and reused. The technology, creativity and will exists – it’s a question of harnessing the right process for the right type of waste and executing it well.’
If this approach is successful, Morrisons plans to roll out the zero waste store format in all of its 498 stores across the UK next year.
>> Interested in reading more about sustainability and the environment? Check out our blog archive.
Stamping out single-use plastics
The government has also issued its latest battle cry in the war on plastics. Having defeated plastic straws, stirrers and cotton buds, it has turned its attention to other single-use plastics.
Single-use plastic plates, cutlery and polystyrene cups are among the items that could be banned in England following public consultation.
The humble cotton bud has now been retired from active service.
Somewhat surprisingly, it estimates that each person in England uses 18 single-use plastic plates and 37 single-use plastic items of cutlery each year; so, it has begun moves to cut out this waste stream.
Environment Secretary George Eustice said: “We have made progress to turn the tide on plastic, banning the supply of plastic straws, stirrers and cotton buds, while our carrier bag charge has cut sales by 95% in the main supermarkets. Now we are looking to go a step further as we build back greener.”
All in all, it’s encouraging to see that companies and the government are brushing up on their sustainable practices.
>> Curious to find out what the future looks like for lab-processed food and meat alternatives? Read what the experts say here.
Life is busy for Rhys Archer. Outside of her work as EPSRC Doctoral Prize Fellow in Biomedical Materials at the University of Manchester, she founded Women of Science to share stories about real women working in science. She has championed STEM in schools in her spare time and received the Robert Perrin Medal from the Institute of Materials, Minerals, and Mining – all before her 30th birthday.
Rhys is also refreshingly forthright in her views. She took the time to speak to us about everything from attitudes towards disability in academia, the problem with STEM statistics, and finding that sense of belonging in science.
Would you mind telling me about your work at the University of Manchester and the research areas that interest you most?
My research interests have always been interdisciplinary – I am a bit of a magpie when it comes to research and I get excited by projects in different areas. Luckily, being a researcher in materials science means that I can apply my knowledge and skills in a wide array of areas and industries. I have recently finished my doctoral studies looking at how carbon fibre composites are damaged during impacts, and how to toughen them while keeping composites light weight, which is particularly useful in the aerospace industry. However, I have since moved over to research in biomedical materials, specifically within tissue engineering, where I am researching biocompatible composite scaffolds for tissue regeneration.
You set up Women of Science in 2016 to share stories about real people in science. How has this been?
When I set up Women of Science, I first looked at it as a personal project that could be of use in schools to young people. However, it became apparent fairly quickly that access to relatable role-models in STEM was needed, not just in schools but also for women across the STEM industry.
Since then, we have been fortunate to be awarded funding to grow the work we do and expand our audiences. One of the most important actions I have taken with Women of Science is to set up an advisory board (which includes a diverse range of women) to share ideas and to influence the direction and activities of Women of Science.
As well as the impact on others, Women of Science has had a huge impact on me personally. When I set up Women of Science I was going through a difficult period of feeling isolated, and found it difficult to feel a sense of belonging in science and in research. By reaching out and hearing other women’s stories – not just their achievements, but also their doubts, worries, and difficulties – I found that I did belong in STEM. I just had to search for it.
Would you mind sharing some of the successes and challenges you’ve experienced in your own career?
At 29, towards the end of my PhD, I was diagnosed as autistic. Looking back, I can see that the challenges I faced, particularly because of depression, anxiety, and isolation, were due to my needs not being considered or met. Being disabled in academia is an ongoing challenge. It is still a fight to gain equitable working arrangements, opportunities, and acceptance.
However, I can also see how the successes I have had, such as setting up Women of Science, and being a part of other projects are a result of ‘being different’. My strongest quality is a diversity of perspective and experience and an eagerness to be a part of a range of different projects.
>> We’re keen to hear diverse perspectives from people working in the chemical industry. Get in touch with us at: eoin.redahan@soci.org
You have championed inclusivity in STEM. Do you think academic institutions and other workplaces could be more inclusive?
Yes. I think there is a huge amount of awareness and conversation about inclusivity in academia and industry, but not nearly as much action and intervention. Often I see workplaces with inclusive policies, but with little consideration of monitoring, evaluating, or reconsidering those policies. We must move past equity, diversity, and inclusivity being a checkbox exercise. The issues faced by women in the workplace are intersectional and complex, and so require well considered, complex solutions.
According to WISE, women now make up 24% of the STEM workforce in the UK. It estimates that this number could rise to 29% by 2030. What do you think about these figures?
While the number of women in STEM is a common metric when considering equality, this does not accurately portray issues surrounding inclusion and belonging. How are women treated? Do they have the opportunity to advance? Are there equitable policies and measures in place? This is particularly true of women in STEM who identify with other protected characteristics around race, disability, sexual orientation, and class. Once you dig into the statistics (where available) further, it is clear that the numbers given are not sufficient to describe the current situation for all women in STEM.
Also, the ‘leaky pipeline’ model is often considered, that is, that the number of women in STEM fall as we follow the statistics from school, to university, and onto the workplace. However, what is not always considered is that, as with a leaky pipeline, when more women are added, rather than ‘fixing’ the pipeline, the cracks become more obvious. Eventually, we reach a point when the pipeline is fractured. We must focus on repairing these cracks, not just increasing a numerical metric.
Additionally, in this current climate, it is incredibly difficult to make predictions as to what the future holds for the number of women in the STEM workforce. A couple of years ago, we could not foresee the impact that a global pandemic would have on women. When we consider the possible effects of climate change over the next decade, can we predict the burden that will be placed on women, or how this will affect women’s choices?
What’s next for you? Are you involved in any exciting projects?
With Women of Science, we have three projects that will be launched towards the end of the year, including a new website, flashcard activities for young people, and a report on the impact of the pandemic on women in STEM. Further ahead, I would love to expand the reach of Women of Science further, working with podcasting and film, as well as reaching out to policy makers. Personally, I am excited to get my teeth stuck into a new research project and see where that leads, as well as doing more teaching, consulting, and any other opportunities that come my way!
>> Are you interested in getting involved in Women of Science? Visit: www.womenofsci.com
A little talked about element, with the atomic mass 140, plays a surprisingly important role in everyday life. It has not only lit many a path, but can be credited with improving and saving the lives of billions of people by enabling cleaner air.
In his talk '140Ce: White light & Clean Air' Andy Walker, Johnson Matthey’s Technical Marketing Director explained why the soft, ductile silvery-white metal Cerium, deserves more recognition.
Walker began by outlining the history of SCI, celebrating its 140th anniversary this year. As an employee of Johnson Matthey, Walker highlighted that George Matthey was among the pioneers of SCI. In addition Walker explained that his PhD research had involved looking at catalysts that included Cerium.
Cerium is a lanthanide and the 26th most abundant element on earth. Indeed it was the first lanthanide to be discovered, found as its ore cerium silicate, in 1803. Cerium makes up 66ppm of the earth’s crust, which is about 5 times as much as lead. It is the only one of the lanthanides able to take on the +4 oxidation state, making it very useful in some of its applications. It is mined in the US, Brazil, India, Sri Lanka, Australian and China, with annual global production of 24 000 tonnes.
However, this straightforward look at the history of Cerium conceals a much more interesting narrative about how this element shaped the life of a number of prominent chemists of the day. Indeed Cerium was found as early as 1751 at a mine in Vestmanland, Sweden by Axel Cronstedt, who also discovered Nickel. Believing it to be an ore of Tungsten, he sent it to Carl Wilhelm Scheele for analysis. However, Scheele was not able to identify it as a new element.
This turn of events for Scheele, perhaps unfairly, helped to seal his moniker as the ‘unlucky chemist’. Scheele, a prominent chemist and pharmacist, had a number of discoveries to his name. He isolated lactic acid, and discovered hydrogen fluoride and hydrogen sulphide.
But as Walker explained, his most notable discovery was oxygen, some three years before Joseph Priestley. Sadly for Scheele; it took him six years to publish his findings, by which time Priestley had already presented his data. Putting a contemporary slant on Scheele’s misfortune, Walker added that the cautionary tale here was that getting things out into the public domain as soon as possible can be important to ensure credit goes to the right people.
Further work by Scheele led to the discovery of a number of elements including barium and chlorine, but sadly he did not receive any recognition because he didn’t manage to isolate them and identify them correctly. The chemist Sir Humphrey Davy did so, some years later, getting the credit for their discovery and isolation.
So it was in 1803 that chemists Wilhelm Hisinger and Jons Jacob Bezelius proved that Cerium was indeed a new element, naming it Cerium after an asteroid/dwarf planet which had been called Ceres. The successful isolation of Cerium took place in 1875, carried out by American chemists William Hillebrand and Thomas Norton, by passing an electric current through molten cerium chloride.
99.95% fine cerium isolated on white background
Once isolated, the earliest application of Cerium was in incandescent gas mantles. Developed by Carl Auer von Welsbach, in 1891, he perfected a mixture of 99% thorium oxide and 1% ceria, which gave a soft white light. Introducing his new mantle commercially in 1892, von Welsbach was able to monetise his development selling his product throughout Europe.
Gas mantles have been replaced, but Cerium’s importance in producing white light remains. As Walker explained, most white LEDs use a blue gallium nitride LED covered by a yellowish phosphor coating made of cerium-doped Yttrium Aluminium Garnet crystals.
In the medical arena, Cerium was used by Sir James Young Simpson, Professor of Medicine and Midwifery at Edinburgh who did a lot of work in the area of anaesthetics. Simpson found that cerium nitrate suppressed vomiting, particularly that associated with morning sickness, and well into the last century, medication containing Cerium could be bought over the counter. In addition Cerium has been the basis of treatments for burns.
Other applications for this versatile element are self cleaning ovens and mischmetal alloy, used in flints for cigarette lighters. Walker shared that the chemist and author Primo Levi, while imprisoned in Auschwitz, was able to steal cerium-iron rods from the laboratory he was forced to work in. Making them into cigarette lighter flints, he was able to barter for bread. Cerium is used to harden surfaces; it is a good polishing agent. Cerium sulphide has been used to replace the pigment cadmium red as a non-toxic alternative and Cerium is widely used across the chemical industry as a catalyst to produce a host of chemicals.
Catalysis is probably where Cerium has impacted most people as the element is the basis for the catalytic converters that have provided cleaner air for billions of people. Walker explained that the driver for the development came during the 1950s when photochemical smog was a problem in the Los Angeles Basin. Measurements at the time indicated that vehicles were responsible for the majority of the hydrocarbon and NOx emissions that led to the polluted air.
This turn of events led researchers to develop systems that could mitigate the emissions. Johnson Matthey was among those doing the early work on catalytic converters. Meanwhile, the automotive industry was pushing back on their introduction, concerned about the costs, durability and effectiveness. Working with Ricardo Engineering, Johnson Matthey carried out durability tests over 25 000 miles which also showed that the catalysts could pass US emissions tests.
The catalysts had to operate in three ways, at the same time, oxidising carbon monoxide (CO) and hydrocarbons (HC) while reducing NOx. Early catalysts, circa 1975, were based on Palladium and Platinum and focused on oxidising the CO and HC. Around 1978 a second catalyst was introduced to reduce NOx.
However, the introduction of Cerium then made it possible to develop a single catalyst that was able to carry out the functions that the researchers had wanted to achieve. Hence, 1981 saw the introduction of the three way catalytic converter with all three reactions enabled over a single catalyst. More recently ceria-zirconia oxide based catalysts have been developed with much higher oxygen storage capacity than ceria.
The impact of these developments has allowed the implementation of much more stringent air quality and emissions standards. Indeed Johnson Matthey estimates that its Cerium-based catalysts are responsible for removing around 40 tonnes of pollutants every minute of every day.
A single element has indeed impacted many lives.
We always hear about athletes eking out that competitive edge through subtle changes in diet or equipment. Well, when it comes to making our buildings more energy-efficient, dozens of different technologies could make a difference. Every one may not be earth juddering on its own, but each could help decarbonise our homes by degrees.
Phase-changing materials (PCMs) may have a role to play in reducing our reliance on power-hungry cooling and heating systems in the home. At Texas A&M University, researchers have developed PCMs to passively regulate temperatures inside buildings.
They believe their 3D-printed phase-change materials - compounds that can change from a solid to liquid when absorbing heat, or from liquid to solid when releasing heat - could be incorporated into our homes in paint or other interior effects to regulate interior temperatures.
New phase-change material composites can regulate ambient temperatures inside buildings | Image credit: Texas A&M University College of Engineering
Their partial substitute to the heating, ventilation and air conditioning (HVAC) systems that predominate in many of our buildings is a light-sensitive liquid resin with a phase-changing paraffin wax powder.
According to the researchers, their 3D printable ink composite improves upon existing PCMs in that it doesn’t require a separate shell around each PCM particle. When the PCM is mixed with liquid resin, the resin acts as both the shell and building material, enabling thermal energy management without any leakage. They use an ultraviolet light to solidify their 3D printable paste and make it suitable for use in our buildings.
“The ability to integrate phase-change materials into building materials using a scalable method opens opportunities to produce more passive temperature regulation in both new builds and already existing structures,” said Dr. Emily Pentzer, associate professor in the Department of Materials Science and Engineering and the Department of Chemistry.
To date, the researchers have only tested their materials on a small scale in a house-shaped model. Nevertheless, after placing their 3D printed model inside an oven, the results were encouraging. The model’s temperature was 40% different to outside temperatures compared to models made using traditional materials.
From solar panels and insulation to heat pumps and phase change materials, much has been done to make our homes more energy-efficient
“We’re excited about the potential of our material to keep buildings comfortable while reducing energy consumption,” said Dr. Peiran Wei, research scientist in the Department of Materials Science and Engineering and the Soft Matter Facility. “We can combine multiple PCMs with different melting temperatures and precisely distribute them into various areas of a single printed object to function throughout all four seasons and across the globe.”
Perhaps we won’t see PCMs in widespread use in our buildings any time soon, but it’s always heartening to see the use of passive heating and cooling systems in our buildings. Anything that contributes to the decarbonisation mix is certainly worth investigating further.
Which technologies will propel industry forward and give companies that competitive advantage? According to digital consultancy McKinsey Digital’s Tech Trends Index, several technologies will have a profound and disruptive impact on industries including the chemical sector. So, which ones will have the biggest effect on the way you work in the coming decade?
1: Automation
By 2025, more than 50 billion devices around the world will be connected to the Industrial Internet of Things (IIOT) and about 600,000 industrial robots a year will be in place from 2022. The combination of these, along with industrial processes such as 3D and 4D printing, will speed up processing and improve operational efficiency.
According to McKinsey, 50% of today’s work practices could be automated by 2022 as ever more intelligent robots (in physical and software form) increase production and reduce lead times. So, how does this change look in the real world?
According to the McKinsey Tech Trends Index, 10% of today’s manufacturing processes will be replaced by additive manufacturing by 2030.
According to the Tech Trends Index, one large manufacturer has used collaborative robots mounted on automatic guided vehicles to load pallets without human involvement, while an automotive manufacturer has used IIOT to connect 122 factories and 500 warehouses around the world to optimise manufacturing and logistics, consolidate real-time data, and boost machine learning throughput.
2: Next generation computing
An almost incredible 368,000 patents were granted in next generation computing in 2020. Advanced computing will speed up the processing of reams of data to optimise research and cut development times for those in the chemicals and pharmaceuticals industries, accelerate the use of autonomous vehicles, and reduce the barriers to industry for many eager entrants.
‘Next-generation computing enables further democratisation of AI-driven services, radically fast development cycles, and lower barriers of entry across industries,’ the index notes. ‘It promises to disrupt parts of the value chain and reshape the skills needed (such as automated trading replacing traders and chemical simulations, reducing the need for experiments).’
According to McKinsey, AI will also be applied to molecule-level simulation to reduce the empirical expertise and testing needed. This could disrupt the materials, chemicals, and pharmaceuticals industries and lead to highly personalised products, especially in medicine.
3: The Bio-revolution
It doesn’t take much investigation before you realise that the bio-revolution has already begun. Targeted drug delivery and smart watches that analyse your sweat are just two ways we’re seeing significant change.
The Tech Trends Index claims the confluence of biological science and the rapid development of AI and automation are giving rise to a revolution that will lead to significant change in agriculture, health, energy and other industries.
In the health industry, it seems we are entering the age of hyper-personalisation. The Index notes that: ‘New markets may emerge, such as genetics-based recommendations for nutrition, even as rapid innovation in DNA sequencing leads ever further into hyper personalised medicine.’ One example of this at work in the agri-food industry is Trace Genomics’ profiling of soil microbiomes to interpret health and disease-risk indicators in farming.
4: Advanced materials
It’s no secret that we will need to develop lighter materials for transport, and others that have a lighter footprint on our planet. According to McKinsey, next generation materials will enhance the performance of products in pharma, energy, transportation, health, and manufacturing.
For example, molybdenum disulfide nanoparticles are being used in flexible electronics, and graphene is driving the development of 2D semiconductors. Computational materials science is another area of extraordinary potential. McKinsey explains: ‘More new materials are on the way as computational-materials science combines computing power and associated machine-learning methods and applies them to materials-related problems and opportunities.’
5G networks will help take autonomous vehicles from tentative - to widespread use.
So, which sorts of advanced materials are we talking about? These include nanomaterials that enable more efficient energy storage, lighter materials for the aerospace industry, and biodegradable nanoparticles as drug carriers within the human body.
These are just four of the 10 areas explored in the fascinating McKinsey Digital’s Tech Trends report. To read more about the rest, visit: https://www.mckinsey.com/business-functions/mckinsey-digital/our-insights/the-top-trends-in-tech
As silicon reaches its solar ceiling, perovskite has emerged as one of the main materials of choice in the next generation of solar panels. Indeed, Oxford PV’s much anticipated perovskite-silicon solar cell could take conversion efficiency well beyond what is currently achieved on the roofs of our homes.
The benefits of perovskite are well known at this stage. It could increase the energy we harvest from the sun and improve solar cell efficiency, and its printability could make fabrication cheaper. However, as with almost everything, there are drawbacks.
According to researchers at the SPECIFIC Innovation and Knowledge Centre at Swansea University, the solvents used to control the crystallisation of the perovskite during fabrication hinder the large-scale manufacture of printed carbon perovskite cells. This is due to the toxicity and potentially psychoactive effects of these materials.
The SPECIFIC team claims to have found a way around this after discovering a non-toxic biodegradable solvent called γ-Valerolactone. They say this replacement solvent could be used without affecting solar cell performance. Furthermore, they say it is non-toxic, sustainable, and suitable for large-scale manufacturing.
Left - solvent normally used to make solar cells, which is toxic.
Right - new green solvent developed by Swansea University researchers from the SPECIFIC project
| Image Credit: Swansea University
‘This solvent problem was a major barrier, not only restricting large-scale manufacture but holding back research in countries where the solvents are banned,’ said research group leader Professor Trystan Watson. ‘We hope our discovery will enable countries that have previously been unable to participate in this research to become part of the community and accelerate the development of cleaner, greener energy.’
As the conversion efficiency of solar panels improves, cost is also key. What if you could create the same solar panels in a more cost-efficient way? That was part of the thinking behind another recent innovation in Singapore, where Maxeon Solar Technologies has created frameless, lightweight rooftop solar panels. These solar panels can be adhered directly to a roof without racking or mounting systems and allegedly perform just as well as standard solar panels.
The new Maxeon Air technology platform from Maxeon Solar Technologies
‘For close to 50 years, the solar power industry has almost exclusively used glass superstrate panel construction,’ said Jeff Waters, CEO of Maxeon Solar Technologies. ‘As solar panels have increased in size, and the cost of solar cells has been dramatically reduced, the cost of transporting, installing and mounting large glass panels has become a relatively larger portion of total system cost. With Maxeon Air technology, we can now develop products that reduce these costs while opening up completely new market opportunities such as low-load commercial rooftops.’
The idea is to use these peel-and-stick designs on low-load roofs that cannot support the weight of conventional solar systems; and they will be rolled out in 2022. Time will tell whether the innovations in Swansea and Singapore have a bearing on companies’ solar systems, but they provide more evidence of the ingenuity that is making solar power cheaper and more efficient.
We’re starting to see those silent cars everywhere. The electric vehicle evolution is gradually seeping onto our roads. Every month or two, we also seem to read about another wind power generation record in the UK, or some super solar cell. Pension funds and big corporations are coming under great pressure to divest from fossil fuels. The clean power revolution is well underway.
And yet the third biggest polluter of the planet - after power and transport - awaits the seismic shift that will shake it to its foundations. Indeed, cement production still accounts for roughly 8% of the world’s greenhouse gas emissions.
The problem is that creating cement is an energy-intense, polluting process with firing temperatures of 2,700 degrees Fahrenheit needed to create it, and plenty of CO2 released during processing.
Green cement and concrete are needed to reduce emissions in construction and other industries.
But there are signs that the processing could become cleaner. A recent report released by Market Research Future (MRFR) predicts that concrete (of which cement is a key ingredient) use could get appreciably greener over the next six years. It estimates that the global green concrete market size will grow at a 9.45% compound annual growth rate from 2020-27.
MRFR attributes this rise to several factors. First, there is a growing demand for green or recycled concrete (that incorporates waste components) within the construction industry. For builders, it enhances their environmental credentials and will increasingly become a business-savvy investment as governments seek to reduce carbon emissions.
Green building codes and the creation of energy-efficient infrastructure will also help propel this growth, and changing building regulations in massive markets including China, India, and the Middle East will result in many manufacturers looking to develop different material combinations. Increasingly, we’re seeing manufacturers turning to less energy-intensive manufacturing methods and investigating which waste materials could be used to create a greener cement or concrete that doesn’t compromise on performance.
Researchers at Chalmers University of Technology, in Sweden, have even been developing a rechargeable cement-based battery. If it ever comes to pass, this could be used to create buildings that store energy like giant batteries. Some manufacturers are also looking into the electrification of kilns, which isn’t feasible yet, and carbon capture and storage has long been mooted as a means to reduce industrial emissions.
Imagine an entire twenty storey concrete building that can store energy like a giant battery. This could be possible if Chalmers University’s cement-based rechargeable batteries come to fruition. | Image Credit: Yen Strandqvist/Chalmers University of Technology
The good news is that we don’t just have people all over the world working on low-carbon materials and manufacturing methods; experts in the UK are tackling the issue right now. On 2 June, speakers at the SCI’s free webinar, Ultra-low carbon concrete, a sustainable future, will examine some of the exciting initiatives underway.
These include an award winning, industry accepted ultra-low carbon alternative to traditional cement, which could result in CO2 savings of up to 78%, and the potential of using offsite manufacturing to provide commercial projects with a sustainable structural frame solution.
As with transport and power, cement is getting greener increment by increment. But with drastic climate change consequences dangling above us like the Sword of Damocles, now is the time for concrete action.
Register for Ultra-low carbon concrete, a sustainable future today at: https://bit.ly/33WfjkN.
Sometimes, when you try to solve one problem, you create another. A famous example is the introduction of the cane toad into Australia from Hawaii in 1935. The toads were introduced as a means of eliminating a beetle species that ravaged sugar cane crops; but now, almost a century later, Western Australia is inundated with these venomous, eco-system-meddling creatures.
In a similar spirit, disposable face masks could help tackle one urgent problem while creating another. According to researchers at Swansea University, nanoplastics and other potentially harmful pollutants have been found in many disposable face masks, including the ones some use to ward off Covid-19.
After submerging various types of common disposable face masks in water, the scientists observed the release of high levels of pollutants including lead, antimony, copper, and plastic fibres. Worryingly, they found significant levels of pollutants from all the masks tested.
Microscope image of microfibres released from children's mask: the colourful fibres are from the cartoon patterns | Credit: Swansea University
Obviously, millions have been wearing single-use masks around the world to protect against the Covid-19 pandemic, but the release of potentially harmful substances into the natural environment and water supply could have far-reaching consequences for all of us.
‘The production of disposable plastic face masks (DPFs) in China alone has reached approximately 200 million a day in a global effort to tackle the spread of the new SARS-CoV-2 virus,’ says project lead Dr Sarper Sarp, whose team’s work has been published on Science Direct. ‘However, improper and unregulated disposal of these DPFs is a plastic pollution problem we are already facing and will only continue to intensify.
The presence of potentially toxic pollutants in some face masks could pose health and environmental risks.
‘There is a concerning amount of evidence that suggests that DPFs waste can potentially have a substantial environmental impact by releasing pollutants simply by exposing them to water. Many of the toxic pollutants found in our research have bio-accumulative properties when released into the environment and our findings show that DPFs could be one of the main sources of these environmental contaminants during and after the Covid-19 pandemic.’
The Swansea scientists say stricter regulations must be enforced during manufacturing and disposal of single-use masks, and more work must be done to understand the effect of particle leaching on public health and on the environment. Another area they believe warrants investigation is the amount of particles inhaled by those wearing these masks.
‘This is a significant concern,’ adds Sarp, ‘especially for health care professionals, key workers, and children who are required to wear masks for large proportions of the working or school day.’
In this new series, members of the SCI Mid-Career group offer advice on career management and how to overcome career challenges.
In our latest interview, we hear from David Freeman, Research & Technology Director for Croda’s Energy Technologies business.
Please tell us about yourself and your career journey.
After a PhD in organic chemistry, I started my career with ICI Paints in Slough in 1998, working in a product development role. Within a couple of years, I moved to another ICI business, Uniqema, and had various technical roles around the chemical synthesis or process development of new materials.
These early roles – and the people I worked with during this time – had a big impact on me in terms of ways of working and how to deal with people. I subsequently joined Croda in 2006 and have since had further technical roles – initially around the technical management of Synthesis programmes in Croda, then technical management of Applications programmes, and finally on to my current role of R&T Director for Croda’s Energy Technologies business.
This last transition was probably the most interesting and challenging as it forced me to think much more strategically about the “what” rather than the “how” and what leadership versus management was all about. I see this area as being hugely important to the Mid-Career group.
What are your keys to managing your career at this stage?
Development remains really important to me from a personal perspective. I have always driven my own development, but been well supported by the organisations I’ve worked for: both by technical management teams and HR teams. At the mid-careers stage, there are lots of important things to think about but I consider the following to be key:
- (i) Self-understanding and feedback: make sure you understand your strengths and weaknesses and how these manifest themselves with colleagues by seeking open and honest feedback
- (ii) Get external perspectives on your areas of interest and expertise. This for me is really key in challenging thinking and bringing new ways of working and innovation to your role
- (iii) Understand the big picture: make sure you’re clear about what’s going on in the world at a high level and the part you and your organisation have to play in meeting these challenges.
What challenges are there around mid-career support?
I feel very fortunate to have worked for organisations where development is extremely important – support is always on hand when I need it. The key challenge is a personal one and it’s about making enough time to focus on the right development areas. We are all busy but if we want to develop ourselves enough, then we will find that time!
Related Links:
We are increasingly conscious of the need to recycle waste products, but it is never quite so easy as rinsing and sorting your waste into the appropriate bins, especially when it comes to plastic.
Despite our best intentions, only around 16% of plastic is recycled into new products — and, worse, plastics tend to be recycled into low quality materials because transformation into high-value chemicals requires substantial amounts of energy, meaning the choices are either downcycling or prohibitively difficult. The majority of single-use plastics end up in landfills or abandoned in the environment.
This is a particular problem when it comes to polyolefins such as polyethylene (PE) and polypropylene (PP), which use cheap and readily available raw materials. Approximately 380 million tonnes of plastics are generated annually around the world and it is estimated that, by 2050, that figure will be 1.1 billion tonnes. Currently, 57% of this total are polyolefins.
Why are polyolefins an issue? The strong sp3 carbon–carbon bonds (essentially long, straight chains of carbon and hydrogen atoms) that make them useful as a material also make them particularly difficult to degrade and reuse without intensive, high energy procedures or strong chemicals. More than most plastics, downcycling or landfill disposal tend to be the main end-of-life options for polyolefins.
Polyethylene is used to make plastic bags and packaging.
Now, however, a team of scientists from MIT, led by Yuriy Román-Leshkov, believe they may have made a significant step towards solving this problem.
Previous research has demonstrated that noble metals, such as zirconium, platinum, and ruthenium can help split apart short, simple hydrocarbon chains as well as more complicated, but plant-based lignin molecules, in processes with much lower temperatures and energy.
So the team looked at using the same approach for the long hydrocarbon chains in polyolefins, aiming to disintegrate the plastics into usable chemicals and natural gas. It worked.
First, they used ruthenium-carbon nanoparticles to convert more than 90% of the hydrocarbons into shorter compounds at 200 Celsius (previously, temperatures of 430–760 Celsius were required).
Next, they tested their new method on commercially available, more complex polyolefins without pre-treatment (an energy intensive requirement). Not only were the samples completely broken down into gaseous and liquid products, the end product could be selected by tuning the reaction, yielding either natural gas or a combination of natural gas and liquid alkanes (both highly desirable) as preferred.
Polypropylene is used in bottle caps, houseware, and other packaging and consumer products.
The researchers believe that an industrial scale use of their method could eventually help reduce the volume of post-consumer waste in landfills by recycling plastics to desirable, highly valuable alkanes — but, of course, it's not that simple. The team says that more research into the effects of moisture and contaminants in the process is required, as well as product removal strategies to decrease the formation of light alkanes which will be critical for the industrialisation of this reaction.
However, they believe the path they're on could lead to affordable upcycling technology that would better integrate polyolefins into the global economy and incentivise the removal of waste plastics from landfill and the environment.
More about the study can be read here:
https://pubs.acs.org/doi/full/10.1021/jacsau.0c00041