There are some things you’ve always been able to depend on: steel is strong, paper is weak and glass is fragile. But not any more. Thanks to scientists, paper and glass can now be stronger than steel, as can light metals like aluminium and even fibres. Not only that, but many of these super-strong materials are also very tough, even though these two properties don’t usually go together.
That scientists are able to produce materials that seemingly defy all common sense is down to the fact that they are now able to study and manipulate materials at the nanoscale, allowing them to gain unprecedented insight into the way materials bend and fracture. ‘Our study on mechanics at the nanoscale has helped us to find the bottlenecks in the design of nanocomposites, which need to be addressed to most effectively enhance the mechanical performance of structural materials,’ says Horacio Espinosa, professor of mechanical engineering at Northwestern University in Illinois, US.
Although the terms strong and tough are bandied about all the time, they have a specific meaning to materials scientists. Strength refers to a material’s ability to withstand applied stress, in the form of being pushed, pulled or bent, without either fracturing or deforming. Toughness refers to a material’s ability to withstand fracturing when stressed.
In a sense, the two terms are related, because they both involve resisting fracture. But strong materials resist fracture solely through the strength of their chemical bonds, preventing cracks from forming. Tough materials, on the other hand, resist fracture by preventing cracks from spreading, often by deforming.
Although some materials, such as bone, are both strong and tough, most non-natural materials tend to be either strong and brittle or weak and tough. Thus, a steel pipe is very strong but snaps at high enough stresses, while a copper pipe is much weaker but bends extensively before breaking. ‘Traditionally, strength and toughness have been mutually exclusive properties in materials,’ says Robert Ritchie, a professor of mechanical engineering at the University of California, Berkeley, US.
In a crystalline material like a metal, the component atoms are arranged in a regular, ordered array. But because we don’t live in a perfect world, this regular array tends to be sprinkled with defects. These defects can include missing atoms, known as point defects, or the presence of an extra line of atoms that push the others aside, known as dislocations.
When stress is applied to a material, it can cause these dislocations to move because the chemical bonds around the dislocation are already under strain and so are the first to break when any stress is applied. Actually, the dislocations don’t really move they just appear to, as the bonds around them repeatedly break and reform, moving the defect through the material. It’s similar to the way a ruck can appear to move along a carpet: the ruck itself doesn’t move, it’s just that different parts of the carpet sequentially adopt the ruck configuration.
Moving dislocations
The effect of these moving dislocations, however, are very real because they allow the crystalline material to deform. The repeated breaking and reforming of chemical bonds around dislocations provides the mechanism by which one plane of atoms in the array can slip over another. This process helps to stop the material fracturing because it takes less energy to move dislocations around than it does to spread cracks. Hence, up to a certain level of stress, the crystalline material deforms rather than fractures.
Now, this is all well and good if you want a tough, bendy material, but if you want a strong, rigid one then you need to stop the dislocations from moving, thereby preventing the material from deforming. Fortunately, there are a number of ways this can be done.
Many metals are naturally quite strong because in bulk form they are polycrystalline, meaning they are made up of lots of tiny crystalline grains. In each grain, the component atoms form a regular array, but the arrays in each grain are at different angles to each other. As a consequence, the grains have distinct boundaries between them and these boundaries can block the movement of dislocations.
Metals can be made even stronger by adding impurity atoms that fit into gaps in the array and which can also block the movement of dislocations. Steel is an alloy of iron, but is much stronger than iron because of the presence of small amounts of carbon – less than 2% by weight.
Taking advantage of their growing ability to monitor and control materials at the nanoscale, scientists have recently begun to refine both of these strengthening techniques, as well as develop new ones. The end result is a suite of new superstrong materials that are giving steel a run for its money.
For example, Zhu heads a team of material scientists that in 2010 reported the development of a new aluminium alloy as strong as steel. This alloy consists of a blend of aluminium, zinc and magnesium that Zhu and his colleagues processed using a technique known as high-pressure torsion, which involves repeatedly twisting the alloy at high pressures.
Using atom probe tomography, Zhu found that this aluminium alloy possesses far smaller grains than normal, with an average size of 20–30nm, compared with a usual size of larger than 1μm. In addition, the zinc and magnesium atoms tend to congregate at grain boundaries, forming a nanoscale network that pervades the entire bulk metal. This all acts to block the movement of dislocations, giving the alloy a yield strength, meaning the stress at which the alloy begins to deform permanently, of 1GPa – making it stronger than the strongest steel.
But that’s not all, because the alloy will also deform, by around 5%, making it fairly tough. This happens even though the movement of dislocations has been completely blocked, which should stop the alloy from deforming. Zhu thinks the explanation for this unusual behaviour is that the alloy gradually builds up a large store of immobile dislocations, giving it a certain amount of ductility along with its great strength. This light but strong and tough alloy could be used to produce medical devices or aircraft parts, and has already been licensed for commercialisation.
Amorphous materials
Another team of US material scientists has combined strength and toughness in a material that is commonly thought of as being anything but – glass. ‘The rare combination of toughness and strength, or damage tolerance, extends beyond the benchmark range established by the toughest and strongest materials known,’ claims team member Ritchie.
In glass, the atoms aren’t arranged in a regular, ordered network, like in a crystalline material, but are instead all over the place, producing what is known as an amorphous material. Although the atoms making up amorphous materials are joined to each other by chemical bonds, they don’t have any long-range order, meaning that they are basically a solid version of a liquid.
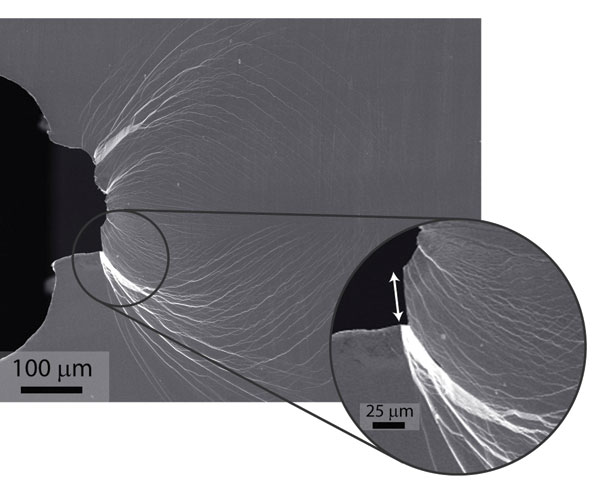
Because they lack long-range order, amorphous materials don’t have to worry about dislocations, making them comparatively strong, but they do have to worry about cracks. For as well as blocking the movement of dislocations, grain boundaries can also stop the spread of cracks. Lacking grains, amorphous materials have no way to stop cracks from spreading, which explains the characteristic brittleness of glass.
This is the case for both normal window glass, made from silica, and metallic glasses, which are made from metal alloys and are commonly found in the anti-theft tags on CDs and DVDs. In 2010, however, Ritchie and his colleagues synthesised a metallic glass consisting mainly of palladium (79%) combined with smaller amounts of gold, phosphorus, silicon and germanium. The scientists employed palladium because of its high bulk-to- shear modulus ratio, which offsets the brittleness of the glass.
In the same way that it takes less energy to move dislocations around than spread cracks in tough crystalline materials, it takes less energy to generate shear bands than to convert those shear bands into cracks in the palladium-based metallic glass. As a consequence, this glass has a yield strength of almost 1.5GPa and a high fracture toughness, meaning that it takes a lot of energy to spread a crack.
Carbon nanotubes
As well as studying and manipulating materials at the nanoscale, materials scientists are also making materials stronger and tougher by combining them with nanoparticles that are inherently strong and tough, such as carbon nanotubes (CNTs). These tiny tubes of carbon can be either single-walled, with a wall just one atom-thick, or multi-walled, in which case two or more nanotubes are stacked inside each other like Russian dolls. But they’re all very strong, with a yield strength of around 100GPa.
Scientists are now looking to harness this strength and toughness by combining CNTs with weaker materials, such as plastics. For example, Espinosa and his team from Northwestern University coated double-walled CNTs with polyacrylate, which binds the CNTs together and orients them in the same direction, and then spun this material to produce a fibre with a yield strength of 1.4GPa that could stretch by as much as 20%. Such tough and strong fibres could be used to produce a new generation of bullet-proof vests.
Nanotubes made from other substances can be just as effective. In 2008, Lars Berglund, from the Swedish Royal Institute of Technology in Stockholm, Sweden, used cellulose nanofibres to produce paper with a yield strength of 214MPa – making it almost as strong as structural steel – and able to deform by 10%.
Cellulose is a central structural component of plant cell walls and is commonly used to produce conventional paper, but this is made up of cellulose fibres around 30μm in diameter whereas the cellulose fibres used by Berglund and his team are just 20–30nm in diameter.
‘The material becomes very homogeneous, with very small defects, compared with a conventional paper network,’ explains Berglund. ‘Cellulose nanofibres are the main reinforcement in all plant structures and are characterised by nanoscale dimensions, high strength and toughness.’ In addition, although the cellulose nanofibres bind very strongly to each other, they are still able to slip and slide past each other, allowing the nanopaper to deform.
To obtain these cellulose nanofibres, Berglund broke down wood with enzymes and then fragmented it with a mechanical beater, producing cellulose nanofibres suspended in water. Draining away the water caused the nanofibres to join together into networks, forming flat sheets of paper.
Since then, Berglund has developed even stronger versions of the nanopaper, with yield strengths as high as 400MPa. He has also incorporated magnetic nanoparticles into the nanopaper to produce a novel form of actuator and used the cellulose nanofibres to reinforce polyurethane.
Materials scientists from Sao Paulo State University in Brazil, led by Alcides Leão, are also reinforcing plastics with cellulose nanofibres, but they are deriving these nanofibres from fruits, such as bananas and pineapples (C&I, 2011, 9, 10). Their aim is to produce light and strong materials for use in cars.
‘The properties of these plastics are incredible,’ says Leão. ‘They are light, but very strong – 30% lighter and three to four times stronger.’
Jon Evans is a frelance science writer based in Chichester, UK.





