BY DAVID BOTT
There is an old saying that “no strategy survives first contact with the enemy” (though I prefer Mike Tyson’s more descriptive version “everyone has a plan until they get punched in the mouth”!). When we were planning the Flue2Chem project, we drew out a detailed Gantt chart with deliverables, dependencies and deadlines. Sadly, the elapsed time between the last post in this series and now is a vivid example that Mike Tyson got it right.
To recap, the aim of the Flue2Chem project is to collect the carbon dioxide from flue gases and turn it into a simple non-ionic surfactant for use in detergents and other consumer goods – potentially eliminating the need to extract more fossil fuel to make them. Key intermediates are dodecanol and ethylene oxide, but the first step is to capture the carbon dioxide.
What has gone before
The idea of extracting carbon dioxide from flue gases has been around for a long time, and has actually been “practiced” since the 1950’s. For a long time, it has been driven by the idea that carbon dioxide emissions can be captured and stored underground, thus avoiding adding them to the atmosphere and causing climate change. Nowadays, if you go to a Carbon Capture Utilisation and Storage (CCUS) conference, the few talks on utilisation mostly talk about the direct use of carbon dioxide either in fizzy drinks or for pumping into greenhouses as a feed to horticulture. We are aiming for something different.
The first need is for a source of flue gases. When we were putting together the Flue2Chem consortium we understood that different sources would have different chemical compositions, so we sought out different types of sources to maximise the spread of data for both the techno-economic and life cycle analyses. There are two paper companies in the consortium, Holmen and UPM. Their carbon dioxide emissions are classified as “biogenic”. Both have biomass combined heat and power plants, built in the days when biomass was regarded by the government as a renewable power source, and subsidised under the renewable obligations scheme that formed part of the 2009 Renewable Energy Directive. They each generate about 1000-1500 tonnes of carbon dioxide a day.
We also had the Port Talbot site of Tata Steel as part of the project. You could classify the carbon here as “used fossil carbon”. Coal is used both as a source of heat and as a reducing agent to turn the iron ore into iron. It is a complex process and so there are many sources of carbon dioxide on the Port Talbot site, some mixed with carbon monoxide In total, they generate about 15000-20000 tonnes of carbon dioxide a day.
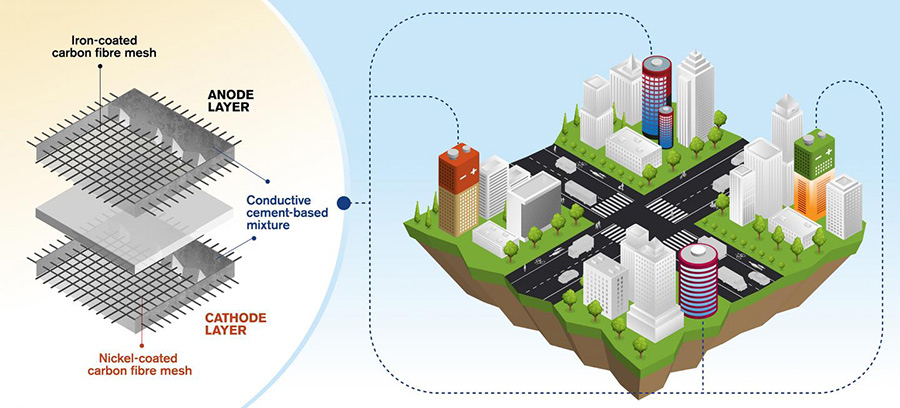
The basic requirement for a process to capture carbon dioxide is easy to state – you need a system that will reversibly absorb carbon dioxide, and some good engineering!
The liquid amine route for capturing carbon dioxide uses a mixture of amines to react with the carbon dioxide to form carbonates. The absorption is usually carried out in a vertical column where the amine trickles down in a packed column and the carbon dioxide flows up. The resulting carbonate is then moved into another column where it is heated to decompose the carbonate to reform the amine and release the carbon dioxide. The energy efficiency of the process is largely determined by the energy required to decompose the carbonate. Over the years, different companies have optimised their mixture to minimise the energy costs and often keep this as “black art”.
Solid state absorption systems rely on physisorption. They used to be based on zeolites, but many recent ones use metal-organic frameworks. The early ones used a similar temperature driven process to control the absorption and desorption, but there are now systems based on pressure swing, where the absorption is driven by higher pressure and the desorption by much lower pressures. These are suggested to use lower energy than the more conventional temperature driven systems.
So, how is it going?
Early on in the project, one of the two companies providing the capture systems we wanted to include in the project – Carbon Clean – ran into an issue with the Environment Agency’s policy regarding solvent disclosure. They use an amine based solvent and, as mentioned above, they want to protect the confidentiality of their IP from this major commercial risk. However, the Environment Agency requires disclosure of any chemicals that might be emitted in any process, and most amines have a measurable partial pressure at the temperature used in the carbon capture process, so although they might have been able to get an exemption for a research or test use, once they go commercial in the UK with their system, they will have to disclose to the Environment Agency. AND, the Environment Agency is subject to Freedom of Information requests and would have to disclose Carbon Clean’s proprietary information. This is why Carbon Clean chose to withdraw its technology from the project, while continuing to provide techno-economic analysis.
This led to another decision – this time by Tata Steel. They had already worked with Carbon Clean in India and were looking to scale up the technology to the 10 tonnes/day envisaged within the project. With that off the table, they wanted to rethink their plans. As they were doing so, the bigger announcement that they would close the blast furnaces and move to use electric arc furnaces at Port Talbot amplified their concerns. Depending on the exact implementation route they choose, there might be minimal emission of carbon dioxide, so they withdrew from the work package to collect carbon dioxide.
Fortunately, in addition to Carbon Clean, we had also included a solid state capture technology, albeit at a much lower state of technology development, in the project. FluRefin had been developed at the University of Sheffield and was being commercialised by Carbon Capture and Utilisation International (CCUI). This had been operated at the small scale but as part of the project, it was being scaled up to 1 tonne/day capture. This required wholly new equipment, some of which had to be imported from India, some from Germany, but was assembled in the UK. It was planned to be installed at the first collection site (Holmen) at the end of November 2023, was actually delivered to site in mid-January, but commissioning issues delayed the first real carbon capture until late April. We have learnt a lot about fast-tracking process development – and the challenges it causes, partners working off different versions of the Process and Instrumentation Diagrams, the design experts being in Sheffield and the equipment being in Workington and so on but, as anyone who has done this before will tell you, this is all quite normal and we were very optimistic in our initial plans! Once on site in Workington, we had the support of some excellent engineers and the various problems were overcome.
One aspect of using a pressure swing process is the need to compress the input gas. This required the use of a number of compressors, but when they arrived we discovered that they had been designed for compressing air to be used as “compressed air” and were a bit “leaky” on the input side. We knew this because the output carbon dioxide concentration was lower that the input and not as we needed and thought we would get. This required more engineering to adapt the compressors for our use.
Another requirement of using the pressure based system is that the input flue gases need to be cooled (from about 150oC to around 30oC) – this recovers a fair amount of heat. More engineering was required!
Over the next few months, we started to capture enough carbon dioxide to supply the chemical conversion work packages. But this led to another “challenge”. We are aiming to capture about 1 tonne per day. This is below the level where we could engage one of the major gas product companies to provide bottling technology, and we were initially not planning to liquefy the gas (so did not have the required equipment). This means we were using a fairly basic “put gas in a pressurised gas bottle” process. Luckily, the University of Sheffield team were also involved in another UK Research & Innovation (UKRI) project – called SUSTAIN Steel. They had a small carbon dioxide liquefaction kit. We have “borrowed” it and are using it to liquefy the captured carbon dioxide, albeit at a very slow rate. We have other ideas for how to do this at a larger scale, but this works, and we are now capturing the required amount of carbon dioxide to send to the two centres where the next stage of our supply chain – the conversion of the carbon dioxide to ethylene oxide and dodecanol – will be carried out, but that’s another post!
So, what have we learnt?
Firstly, that project plans written in a hurry to fit within the proscribed timescale and budget will almost certainly be too optimistic and liable to require drastic adjustment. This was no real surprise to those who had been involved in scaling up processes before, but we rediscovered the saying that “if it can go wrong, it will” is irritatingly true. However, the capabilities of the individual organisations in the project and the creativity of the combined “leadership team” means that we have always found a way out of every “challenge”. We have also applied to Innovate UK to extend the project by 4 months, and have been successful, so have bought a little more time. The next work packages, which would have been squeezed by the 6 month (or so) delays will be “less” squeezed.
Perhaps the biggest learning is the need for flexible resources to enable scaling up the sort of processes we are using. This does not necessarily mean expensive new buildings or plants. A small carbon dioxide liquefaction plant that could handle 1-10 tonnes a day would have saved us about 2 months. More engineering expertise in the consortium might have saved us another couple of months building and commissioning the FluRefin plant. We had some allowance for creep in the original plan but when every month the deadline slipped by a month, I felt sorry for the project manager!
What has really been driven home to us is that the change we are attempting to prove – that it is feasible to move the chemistry supply chain away from virgin fossil carbon as a feedstock – may be scientifically credible, but reducing anything from theory to reality is harder than we think and required even more planning and effort than we imagined.
And we need to use realists, or even pessimists, as planners!!
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
Science is hard work. Understanding the world around us well enough to predict the behaviour of everything from sub-atomic particles to planets requires insight, patience, imagination and rigour. But science also lays the foundations of many of the industries that have changed our world – from pharmaceuticals to airplanes.
It is this application of science to address societal challenges that benefits people. And one of the biggest challenges is moving away from using virgin fossil carbon to feed the chemical supply chain!
It is worth stating at this point that, as we have talked about this work, we meet many people who do not understand how the chemistry using industries underpin other supply chains. We have had to explain many times to disbelieving audiences that cleaning products are currently mostly made from oil!
At the moment, the many branches of the chemistry using supply chains start with about 2.6 billion tonnes of carbon dioxide equivalent produced from oil and gas extracted from the fossil reserves (about 5-6% of the carbon extracted annually goes into this use). There are other sources of carbon and the science to use them is proven, but we need to start development and implementation. This means designing and building manufacturing capacity that operates efficiently to produce the volume of material needed to satisfy the current and future market needs – and this is a lot!
Technology, Development, Innovation – whatever you call the process of turning science into products is also hard, but in a different way. Something may be scientifically possible, but to make a product out of it for less than the price people will pay for it and at a volume that all the people that want it will be satisfied can be difficult. And competing with an established route where the feedstocks are cheap and easily available, and the processes have been optimised and scaled over decades, requires tenacity.
Flue2Chem is an Innovate UK funded consortium of 16 organisations that could make up a wholly new supply that produces the materials we currently use at a large scale but without starting from virgin fossil carbon. Rather than trying to do everything all at once, it is focused on a single product – a surfactant that is widely used in a range of cleaning products. It is one way to re-imagine the future of those parts of chemistry based industries that currently use virgin fossil carbon as a feedstock. The project is focused on demonstrating that carbon dioxide can be collected from flue gases and, by a series of chemical steps, turned into that common surfactant. Although it is focused on a single product, the processes and the learning from scaling them up could be applied more widely.
Flue2Chem consortium members
The challenge of scaling-up
Making a lot of anything usually involves making it in a large factory – and the more product needed to satisfy the market demand, the larger the factory. We are mainly talking about chemistry here, so the core manufacturing unit is a reactor. In the laboratory, most people think that chemistry is done in test tubes, but the truth is that the most common reaction vessel is probably a sub 1 litre round-bottomed flask. This is where the science of the basic reactions is tested and optimised. The next step is then a vessel between 5 and 25 litres in size. This is where we first encounter scaling laws! If we make a reaction vessel which is twice as big in the linear sense, it has 8 (23) times the volume and 4 (22) times the surface area. Most chemical reactions involve either the absorption or emission of heat – the amount of heat (absorbed or generated) increases with the volume of the reaction, but the heat must move through the walls of the vessel. So, a reactor three times as big (the 1 litre to 25 litre example above) generates or absorbs 27 times the heat but it must move through 9 times the surface area. The reactor surfaces must be three times as thermally conductive to allow this. The 25 litre flask is often exactly the same design and made of the same material as the 1 litre flask, so this does not happen!
Given that chemical reactors can have a capacity up to 1,000,000 litres these are substantial size differentials! Here the ratio between the initial reactor and the final, at-scale reactor means the challenges for this simplest reaction parameter must change 1000 times.
And thermal management is not the only challenge. Mixing also requires more energy at larger scales; removing the by-products of the reaction is also more problematic as reactor size increases.
But working at large scale has some commercial advantages – the cost of capital equipment needed usually scales at a lower rate than the science gets hard, and the complexity of the ancillary equipment can also be optimised, making larger factories more commercially attractive. Since big factories are often more commercially attractive, the technological challenges have been worked on for decades and large reactors for the currently used reactions are well optimised!
This optimisation, or scaling-up of the process nearly always goes in steps, and the size of those steps depends on the confidence of those in charge! These days, a lot of these steps can be carried out using computer based process software – decades of scaling things up has given industry experience of and insight into the critical factors that need to be considered, but moving to new reactions means the basic information must be measured again!
And this is just to get it to work!
But how big should you make it? An important factor in determining the size of the reactor is the size of the market – and therefore how much product you need to make.
So, how much do we need to make to prove this new supply chain will work?
For most people, the scale of the chemistry based industries are not even recognised, let alone considered.
At present the petrochemicals industry, which provide the bulk of feedstocks to the chemistry based industries globally uses about 2.6 billion tonnes of carbon dioxide equivalent a year. To give an idea of scale, if all that was made into polyethylene (commonly used in packaging, toys and cars) it would be a cube just under a kilometre along each length.
As already described, the target molecule of Flue2Chem is a simple surfactant with a chain of 12 carbon atoms as the oleophilic (the bit that attaches to the dirt) end and 5 to 7 ethoxy units as the hydrophilic (the bit that dissolves in the water) end. It is widely used for all sorts of cleaning products – globally figures of around 17,600,000 tonnes a year are quoted. This is equivalent to about 44,000,000 tonnes of carbon dioxide equivalent – so about 1.7% of the global petrochemicals market! What sounds a lot rapidly becomes small when you look at the actual numbers!
And how is it playing out for the Flue2Chem supply chain?
When we started the project, we had two carbon capture streams – an established liquid based system capable of collecting 10 tonnes/day and a start-up solid state system with a capacity of 1 tonne/day. Given that we also had three sites from which to capture carbon dioxide and the plan was to run each for 30 days, that would have given us an input of just under a 1000 tonnes of carbon dioxide.
At the other end of the supply chain, the final assembly of the surfactant could be carried out at about 1000 tonnes and the consumer products companies could use all that output to carry out test marketing of at least three products.
We rapidly discovered that the bottleneck would be the chemistry to turn carbon dioxide into ethylene oxide (for the hydrophilic end of the surfactant) and dodecanol (for the oleophilic end). The thermo-catalytic routes might be able to make about a kilogramme of the ethylene oxide and probably much less of the dodecanol. And on the biotechnology side of the options, although there were companies making ethanol (a precursor to ethylene oxide) from flue gases, they were not using carbon dioxide as the source. Biotechnology routes are often attractive because they make a single product, but using the biobased route to dodecanol looks like it would be hard to make even a kilogramme.
This would give us a few problems, but as the project progressed, we ran into another challenge. The company with the liquid system decided to withdraw from active carbon capture in the UK (the subject of a later blog), and one of the carbon sources got a government grant to radically overhaul their plant to drastically lessen the amount of carbon dioxide they might produce, so they have “paused” their direct involvement in that part of the project. We had now gone from a capacity of 990 tonnes to 60 tonnes. This was still more than enough to keep the chemists happy, but it gave us another opportunity to be innovative. The two carbon dioxide sources were in Cumbria and North Ayrshire, but the chemistry to convert it was being carried out in Sheffield and Germany and the biology in North Yorkshire. How could we transport that amount of carbon dioxide? At about 1000 tonnes, it might have been possible to “rent” a bottling facility and install it at the capture sites. At less than 100 tonnes, the equipment did not even exist! The solution we are adopting is to pressurise about 3 kilogrammes of carbon dioxide in pressure vessels and transport it from site to site. This limit on how much carbon dioxide we can transport from the emitters to the converters puts another size restriction in place, so a sizable fraction of the carbon dioxide we capture will have to go back into the flue! This is not ideal, but given the goal is to validate a wholly new, more sustainable supply chain and the lack of any other options, we don't have much choice.
There will be more…
Stepping stones to wisdom – what have we learned?
Perhaps we should have checked our numbers before we submitted the proposal, but since any scale-up project is designed to see how difficult it is to scale-up, discovering that there are issues beyond the technological is good learning.
The chemical and biological restrictions are a result of the lack for scale-up facilities in the UK, and we currently have no other options. If we are to develop the new chemistries required to switch feedstocks away from virgin fossil carbon to carbon dioxide, waste biomass or recycled plastics and oils, we will need several levels of scale beyond those currently available.
Underpinning this last point is the fact that many do not understand how the chemistry using industries underpin other supply chains – even their own. We have had to explain the range of products made from virgin fossil carbon (IEA figures are plastics packaging 36%, upholstery, carpets and paints 16%, textiles 15%, home and personal car products 10%, pharmaceutical and agricultural products 11%, car interiors and tyres 7% and electrical 4%) many times and often find ourselves trying to convince disbelieving audiences that cleaning products are currently made from oil! This means that many in government do not see the need to invest at the national scale in them in a coordinated manner. Scaling-up chemistry is generally not well catered for.
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
There is much talk of “decarbonisation” these days, and you could be forgiven for thinking this means eliminating carbon from all human activities. But there are lots of things that need carbon to exist. The chemistry of carbon is unique and nature relies on it for many things. Carbon has direct influence on conditions for life on our planet, whether or not you factor in the specific needs of human beings! And we depend on carbon chemistry for life too – our bodies burn carbon based fuels ourselves (grains, vegetables and meat are all carbon based materials).
We have also depended for millennia on carbon-based materials as clothing (skins, cotton and wool), housing (wood and thatch), and much more – until about 6000 BC when we started using inorganic materials (metals!) as well. With the birth of the oil age in the late 19th century, we started to make synthetic materials, initially striving to reproduce the properties of the natural materials we were used to – polyester was a cotton like material, polyamides were meant to be like silk, polyacrylonitrile was similar to wool in properties. In healthcare we started making naturally occurring therapies (like aspirin and morphine) at scale using synthetic chemistry. As we understood the relationships between chemical structure and properties during the 19th century, we moved on to more complex chemical structures. We cannot get away from carbon because so many planetary systems depend on it, so getting it rid of it altogether is not really an option!
So, what do we mean when we say “decarbonisation”?
The problem is not with carbon dioxide as such, but with the amount we have put into the ecosphere over the last 150 years or so years and the effect it is having on the climate.
In the past there was a lot more carbon than we currently have in the atmosphere – somewhere between 15-30 times as much – but then life wasn’t the same in the Mesozoic period – evidence suggests the average global temperature was 10-15 degrees higher, so species like us would probably not have survived. Over the intervening 150,000,000 years, a variety of natural processes absorbed and sequestered that carbon in rocks, coal, oil and gas. But coal, oil and gas are an easily available source of energy, and their extraction has powered progress over the last 200 years! And when we burn them, the carbon that was previously safety locked up in the ground goes back into the atmosphere.
So what is the problem?
The amount of carbon dioxide we have generated over the last 150 years can be found in the atmosphere, the oceans and on land. This is the product of the coal, oil and gas we have extracted and burnt over the same time period. The problem is that the Earth’s climate cannot cope with this increase, and we need to stop.
Here is how it all adds up:
Atmosphere – Since the beginning of the industrial revolution, the amount of carbon dioxide in the atmosphere has increased from around 2.2 to 3.3 trillion tonnes – meaning we have added about 1.1 trillion tonnes.
Oceans – There is a lot of focus on carbon dioxide in the atmosphere and the impact it has on climate, but carbon dioxide is also absorbed by the oceans, where it also has an impact – the water becomes more acidic and damages marine ecosystems. For every tonne of carbon dioxide in the atmosphere there is about 0.75 of a tonne in the oceans. This means there is probably about 800 billion tonnes in the oceans.
Land – There is also work that indicates that about 8.3 billion tonnes of plastic waste have been produced since 1950 – roughly equivalent to 26 billion tonnes of carbon dioxide. Since plastics account for about 30% of the petrochemical products stream, we can work out that 87 billion tonnes of carbon dioxide from the use of oil and gas as a feedstock is also in the ecosphere.
Total – This means we can estimate that we have put about 1.99 trillion tonnes of carbon dioxide into the ecosphere since we started using coal, oil and gas as a source of energy.
The amount that we find in the ecosphere is almost identical to the amount extracted from the geosphere over this time period. Evidence that this is a man-made effect can be seen by comparing this number to the 2.2 trillion tonnes of carbon dioxide equivalent that industry records show the coal, oil and gas industries have extracted from the planet over the last 150 years. The small difference is probably due to the accuracy of the figures, which have been collected over 150 years!
We often fall into the trap of differentiating between fossil carbon and biogenic carbon, as if biogenic carbon is acceptable, but we have been putting fossil carbon into the atmosphere for so long that it makes up about 35% of the carbon in the atmosphere and has therefore been absorbed by plants and will make up roughly the same fraction of what we call biomass! This means that 35% of the biogenic carbon is really second generation fossil carbon. This becomes important when one source is favoured over another by regulations.
The goal is not to stop using carbon – it is to stop taking it out of the ground and putting it into the ecosphere!
If the various government promises to achieve Net Zero are kept and we do manage to stop using fossil carbon as a fuel by 2050, where will we get the carbon we use as a chemical feedstock that we have come to rely on as the source of materials such as plastics, fertilisers, packaging, clothing, digital devices, medical equipment, detergents or tyres? Currently we use about 2.6 billion tonnes of carbon dioxide equivalent a year to make a wide variety of things – through a “petrochemical” supply chain. We cannot “decarbonise” that without inventing new materials to deliver all of the products, but we can “defossilise” it – which means we stop using virgin fossil carbon as its feedstock.
Where will the carbon come from then?
There are three sources of carbon (biomass, recycled plastics/oils/solvents and carbon capture and utilisation) widely touted as replacements for all that fossil carbon. All are essentially recycling previously used carbon. They tend to get framed as competitors for the role, but if you analyse the amounts available, the likely costs of using them, and the timescales needed to get them to the right scale it looks like we will need them all!
Back to Nature
Biomass refers to using biological sources for the raw materials – wood, crops, animals and bacteria are good examples. Advocates rightly point out that many synthetic materials are clones of natural materials. They tend to forget that society moved to synthetic analogues because there wasn’t enough to satisfy societies needs in terms of volume and price, but global supply chains were less sophisticated back then.
However, from the generally accepted numbers, there would be enough raw materials – the planet produces about 50 billion tonnes of biomass a year – of which about 14 tonnes is produced by man. Of that, it is estimated that about 1.8 billion tonnes of “waste” is produced a year – mainly cellulosic. This produced in farms and food processing plants. Of the biomass that goes to food, we waste about 30-40%, so can add another 1.6 billion tonnes, but this is produced in restaurants and houses, so collection might be expensive! And finally, we have the waste we humans and our animals directly produce. Estimates vary but are usually about 500 million tonnes of human waste and almost 3 billion tonnes from farm animals! There would be enough carbon in biomass to satisfy the requirements, but the logistics of collecting it might be a challenge (and probably involve the generation of more carbon dioxide – there is always a trade-off where transport is involved). Also, biological processes can often be slow and less atom efficient than the currently used chemical processes, but this can be accommodated by appropriate systems design.
Nevertheless, the use of biomass as a feedstock for chemicals is already here and growing in scale.
Second time around
All the carbon that currently goes out in the form of products could form the basis of a different recycling route – often called “chemcycling”. Here the previously used plastics, oils and solvents can be partially broken down into reactive chemical species like those used in the manufacture of the original products. This is usually done with heat, and so costs energy. It also often uses solvents or extra chemistry. Given the range of materials that will make up the input, there will probably need to be a sorting process at the front end. And, like the waste biomass, the input materials will be collected from geographically distributed sites, so will need to be transported to plants where the recycling will take place (with the potential carbon dioxide emissions as a result).
Mining the problem
Finally, we could use the largest source of carbon already in the ecosphere – the carbon dioxide we have been putting into the atmosphere. With its current concentration of about 0.05%, it is a daunting task. However, we can “practice” how to make it work with a higher concentration (often 10-15%) – when we burn things, we produce a gas stream with a higher concentration of carbon dioxide and send it up a chimney or flue. We will probably go on burning things for a few years yet and using them as a source of carbon will not only moderate the amount we put into the atmosphere in the short term, but also enable us to refine the technology so that we can one day we can remove carbon dioxide from the atmosphere and reverse the damage we have done to the climate – although sadly it would take hundreds of years to do so.
Are we there yet?
All three routes to providing a non-virgin fossil source of carbon are being worked on already. However, they are all at such a small scale it is difficult to judge their efficiencies and effectiveness.
- Biomass is probably the most technologically advanced, but scaling-up the processes and addressing the collection and logistics issues are important challenges that need to be overcome.
- Recycling the “second generation” carbon cannot supply enough – it is limited by the efficiencies and costs of all the processes from collection to chemical recycling but can probably contribute a sizable fraction of the requirement.
- Carbon Capture and Utilisation (CCU) is the least well advanced but with a roadmap to Direct Air Capture (DAC) (the slightly different name for capturing the carbon dioxide direct from the atmosphere) offers the most compelling argument for investment – if DAC can be made to work commercially, it can be used to remediate the atmosphere. Net zero targets aim to take us back to 1990 levels of carbon dioxide in the atmosphere by eliminating the use of fossil fuels. Depending on the scale and speed at which DAC can be implemented, it might be possible to get back to pre-Industrial Revolution atmospheric carbon dioxide levels in several hundred years!
Carbon Capture (CC) has rather had its reputation tarnished by the pervasive suggestion that it can be used to capture (and store) carbon dioxide so effectively we can go on using fossil fuels for many years. Most analysts outside the oil and gas industry do not see how the maths works!
All of the routes are years away from making a major contribution to replacing the volume of carbon based feedstocks used to make the wide variety of products (and the market is growing at over 5% per year, which means that it will double in size every 12 or so years). We need to start soon and grow quickly to make any meaningful impact.
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
One of the questions we often get asked is ‘how did you assemble such a large project so quickly?’ The short answer is ‘we didn’t, it took years!’, but the story is important
In the last blog we discussed the scale of the use of petrochemicals to make things society doesn't know are made of fossil carbon, and the drive to change this – this time we will look at how we built the team.
On page 17 of the Chemistry Council Strategy published in 2019, you will find the phrase ‘Sustainable Materials for Consumer Products’. This is (we think) the first time the phrase was used in this context,. and so was effectively the beginning of the story.
Technically, there is prehistory. The UK government had tried twice before to understand and support the “chemistry-using” industries but had failed to capture the breadth and scale of their impact on the economy in their engagement. They had included the pharmaceutical companies alongside the more traditional “chemicals” companies, but not the extensive downstream activities that used chemicals to deliver a product or process. In 2018 they had spread their net a bit wider and included the consumer companies who use chemistry to make a large number of products for a whole range of markets. These companies were seemingly more aware of the way consumers were looking at the discussion on climate change and who were wondering why the products they could not live without had to be made from fossil carbon and contribute to climate change. So, these companies started making commitments to be fossil carbon free by 2030 or not long after. The scale of their production (often millions of tonnes) meant that the necessary changes to their supply chains had to be at a similar scale and they quickly found that their suppliers (and their suppliers) could not easily accommodate the changes they wanted to see in their chosen timeframe. What was required was a more radical rebuilding of their supply chains.
The Society of the Chemical Industry (SCI), who were responsible for the Innovation Committee of the Chemistry Council, decided to build a community who could do something about this challenge.
The first workshop in the area was held at SCI on 22 February 2020. It included participants from retailers (Marks & Spencer, Sainsbury’s, Walgreens Boots Alliance and Enkos), consumer products manufacturers (Unilever, GSK, Reckitt Benckiser and Proctor & Gamble) a number and variety of chemical manufacturers (Croda, Innospec, Johnson Matthey, Thomas Swan, BASF, INEOS, Lubrizol and Synthomer), universities/RTOs (CPI, University of Bath and University of York) and several companies engaged in the recycling of materials (Biffa, Symphony Environmental).
The discussion was outcome-oriented, and we finished the day with a draft list of things to work on in the second workshop. But within a few weeks COVID happened and we went into lockdown! As they learned to cope with Zoom and Teams, the core team met online and developed the ideas from the workshop. First, the tasks were sorted into three main buckets – engagement, new chemistries and measurement.
Talking to people
Under the engagement banner, we recognised the importance of explaining what we understood were the challenges and how best to address them. Audiences would include chemistry-based companies in the supply chain, regulators and policy makers in government, and consumers. Initial attempts at engagement rapidly taught us that before we could explain what we were doing, we often had to make the audience aware of the ubiquity of carbon-based chemistry in everyday life and the relationship to climate change. One example was when I found myself being interviewed by Rachel Johnson on LBC about the impact of laundry on climate change and her not believing that the cleaning products themselves were made from fossil carbon resources!
The idea of starting with awareness raising, moving on to (gentle) discussion of the science and technology before even mentioning the “ask” became our approach!
Changing the way we do things
The new chemistries banner was an area where we were all more comfortable. If we were to avoid using fossil carbon as a feedstock, we needed another source of carbon, or we needed to find the functionality the businesses needed using another element. The second option looked very difficult – and therefore expensive. The options for the first were well known – “biomass” (ill-defined but very popular as a choice), recycled (second generation?) plastics and oils (logistics is the current issue) and carbon dioxide (lots of it about, but more energy needed to get it to a useable state).
Then we agreed (being mostly from business) that we needed a specific target at focus on.
If we could start with a non-fossil source of carbon and turn it into a useful molecule, we would have demonstrated that there was a completely different way of making things. In the process we could learn what was possible, what impact this alternative would have on the environment, whether it was commercially viable and what social impact it might have.
Since there are so many things made of fossil carbon – packaging, paints, adhesives, textiles, carpets, upholstery, tyres, drugs, fertilisers, insulation and cleaning products – selection was difficult, but the team knew a lot about cleaning products, so we started there. After a lot of swapping of anonymised data, we alighted on non-ionic surfactants. They are extensively used in consumer cleaning products (about 1,000,000 tonnes a year) and they are relatively simple molecules. These are made up of a hydrophobic end (usually a 12-14 carbon atom chain) and a hydrophilic end (usually made up of 5-7 ethylene oxide units).
Alongside the search for a way to make this sort of chemical without using fossil carbon, we realised that cleaning products were almost invariably flushed down the drain – and that often that meant straight into streams and rivers. We knew from other studies that they would degrade into other chemicals, and eventually into carbon dioxide – but we could not find any studies that mapped the complexity of that degradation pathway – was it a biological process, or was it photolytic, or a mixture of both? We talked to BBSRC. We talked to NERC. To date we have not been able to interest any UK funding agency in researching this aspect of what happens to these carbon based molecules in the environment, although there is lots or research into the effect of the chemicals on the environment!
Measuring what was going on
The third area we pondered over was how to measure what was going on – both currently and as a result of anything we might do. We looked at various life cycle analysis studies and quickly realised there were real differences between academic studies – and even consultancy or in-house techniques. Given that we knew we would be building a wholly new supply chain, how were we going to measure the impact of our actions?
Getting on with it
We knew better what we had to do. The next problem was to find a way of funding the work. It took almost a year of talking to the KTN and Innovate UK before we became aware of the Transforming Foundation Industries competition. In the late Summer of 2021, we decided to develop a proposal to turn carbon dioxide from flue gases into our target surfactant.
The specification of the competition meant that we had to work with other foundation industries. Given that we wanted a source of carbon dioxide, and they mostly produce it, this gave us options. We built links to paper companies such as Holmen and UPM. They often use biomass boilers as heat and energy sources. These are currently classed as zero emissions under the emissions trading scheme, but the rules are tightening and are looking for the next technology to remain viable. We also talked to Tata Steel, who use coke both as a fuel and reducing agent in their blast furnaces. We tried to talk to cement manufacturers but were unsuccessful.
Next, we needed a means to capture the carbon dioxide in their flue gases. There are basically 3 ways to capture carbon dioxide, solvent adsorption, solid phase adsorption and membranes. We contacted Carbon Clean (who use solvents) and were already talking to the University of Sheffield (who were developing a solid phase process). Both were interested and so joined the consortium.
Turning carbon dioxide into the C12 fatty alcohol (to make the hydrophobic end of the surfactant) and the ethylene oxide (to make the hydrophilic end of the surfactant) was the next goal. The BASF, University of Sheffield and Johnson Matthey all had expertise in thermo-chemical processes which could achieve this and all were interested. We knew there would be biochemical processes that could achieve the same goal but no companies in the UK, so we turned to the Centre for Process Innovation (part of the High Value Manufacturing Catapult) and found they were working in the area so added them to the consortium.
Croda were already the supplier of choice to react these two components together to make the final surfactant.
We then had the consumer product companies, Unilever, Proctor & Gamble and Reckitt, who would evaluate the surfactant and “prove” that getting the carbon from a different source did not change the properties.
We also folded in our concerns about measuring the impact of what we had done by including the UKRI Interdisciplinary Centre for Circular Chemical Economy to evaluate the life cycle and technico-economic aspects of the various processes we were evaluating so that we could put together a justified case for a new supply chain at the end of project.
The Confederation of Paper Industries also joined to be able to understand what we had done and recommend it to other paper companies.
And the SCI joined to help with exploitation and dissemination as the project progressed.
Are we there yet?
We submitted our Expression of Interest to Innovate UK just before Christmas 2021 and (mostly) didn't worry about it until the New Year. On 10 January, we learned that our application was successful, so buckled down to writing the full application.
The consortium submitted its proposal on 6 April 2022
As we understand it there were eight proposals submitted but only money for four, but we were still disappointed that the initial funding announcement did not include us! However, somehow Innovate UK found some more money and the remaining projects were judged to be fundable so on 14 July we learned that we had the money!
For those who haven’t applied for Innovate UK funding before, the detailed process is simple but rigorous. One really, really important step is for every company to sign a “Collaboration Agreement”. This sets out the goals of the project, how it will be managed, and how the intellectual property that arises during the project will be allocated. It is often a source of contention, and the amount and intensity of the contention seems to scale geometrically with the number of partners in the consortium. Given that many of the partners were more used to competing than collaborating and they all had lawyers, it seemed at times to scale exponentially! However, because the scientific leads in all the companies had worked together (some, at this point, for over 3 years) and there was real commitment to get started, (after a small hiccup) we all signed the agreement just before Christmas 2022. It was then that we discovered we all had to sign the Grant Offer Letter as well, but we managed that too.
Then the work started…(see Part 3)
The exam question
So, how did we assemble the consortium?
- It took time, and lots of interaction. The core of the consortium – Unilever, P&G, Reckitt, Croda, BASF, Johnson Matthey and the SCI – had all been sharing ideas, challenges, opportunities and frustrations for over 3 years. Individually some had also worked with the others we invited to join, so there were always personal recommendations and shared experiences.
- It took transparency and honesty. The relationships we have built over those years are strong. We have disagreed (a fair amount) on the way, but we have always shared – and even strengthened through our interaction – a commitment to move from fossil carbon feedstocks to a sustainable chemistry based industry.
- It required shared commitment, an understanding of how the different organisations fitted into what will be a wholly new supply chain, and tireless advocacy both within our own organisations – and to anyone who will listen outside them!
- It needed the patience of a saint!
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
BY DAVID BOTT
A collaboration of 15 organisations is part way through an Innovate UK funded project to turn the carbon dioxide in flue gases into non-ionic surfactants for cleaning products. Many have asked: why we are doing it? how did we build the team? and how things are going? This is the first part of that story.
No one really thinks about the role of chemistry in society. Although everything around us is chemistry – life itself, the natural world, and the material world we have created to add to the natural world – we mostly talk about “chemicals” in a derogatory way. Does society understand how much it relies on carbon-based chemicals? Does chemistry need a PR agency?
Why we need carbon
It is difficult to say exactly when the “synthetic” world started, but the huge expansion came with the use of fractions of the oil and gas we were extracting from the ground as a fuel as material feedstocks about 120 years ago. The petrochemicals supply chain now accounts for about 2.6 billion tonnes of carbon dioxide equivalent a year (about 5.7% of the carbon extracted) and is growing at about 8% a year – therefore roughly doubling every 10 years. And the products at the end of the many supply chains that start with these fossil feedstocks range from things we cannot do without (such as disinfectants, soaps, textiles for clothes and so on) to things we “like” to have but would probably fight to keep (such as cosmetics and flying)!
The focus is often on single-use plastic packaging, which makes up about 30% of that 2.6 billion tonnes. But there are many other products that depend on the same source – products for use in the home (carpets, upholstery, paint and adhesives) at about 16%, textiles (mostly for clothes) at about 15%, pharmaceuticals, agrochemicals and fertilisers at about 11%, cleaning products and cosmetics at about 10%, the interiors of cars and their tyres at about 7% and the use in electrical and electronic products (both insulation and cases) at about 4%. Such is society’s use of these products, that we cannot simply stop using them, or make them from something other than carbon. We will need a source of carbon at the same scale.
At this point in the narrative, it is worth pointing out that although many uses of fossil carbon as a fuel can be replaced by alternative technologies, aviation fuel is different. Although there are prototype electric planes for short haul flights, and visions of hydrogen powered planes at some point, the options for long haul air travel look distinctly limited – with really only Sustainable Aviation Fuel as a runner. The aviation industry would need about 1.3 billion tonnes of carbon dioxide equivalent a year to keep going at their current rate, so about half that needed to supply our material needs. It faces the same challenge as the carbon based materials – if they do not use fossil carbon, where would it come from?
Where we can get carbon from
The source that gets talked about a lot is “biomass”. At the global level, it is estimated that about 100 million tonnes of biomass is produced each year, roughly half on the land and half in the sea. This equates to roughly 200 million tonnes of carbon dioxide equivalent. Most reviews discount the marine biomass as difficult to harvest and then calculate that of the land based only about 8 million tonnes (so, 16 million of carbon dioxide equivalent) a year can be harvested “sustainably”.
Another factor often quoted is the logistical cost of harvesting, since for some crops the energy required to harvest compromises the efficiency of the process. The factor that doesn’t get talked about much is the difference between the biological building blocks and the petrochemical ones we are used to – which would require us to build a different set of supply chains and result in products with different properties.
The second source is the materials made of fossil carbon we have already extracted. There are various estimates of how much plastic is in landfills, but the average seems to be about 5 billion tonnes of plastic – about 15 billion tonnes of carbon dioxide equivalent. This would need to be chemically recycled back to feedstocks equivalent to those that underpin the current supply chains.
The third source is the carbon dioxide we have been “storing” in the atmosphere. Since the start of the industrial revolution when our emission of carbon dioxide started in earnest, we have emitted close to 2.2 trillion tonnes of carbon dioxide – half of which is in the atmosphere and the rest has partitioned into the oceans. The problem is that it is very dilute, so you would have to capture a lot of “air” to get meaningful amounts of carbon dioxide – but people are trying!
However, since we are still burning a lot of fossil fuels (and biomass) at the moment, we have flues and chimneys where the carbon dioxide concentration is many times higher. Why not start there and increase the process efficiency with experience?
However, there is a challenge with using carbon dioxide as a source for carbon-based materials. Carbon dioxide is where carbon tends to end up in an oxygen-rich environment – particularly where there is light. It is basically at the bottom of a thermodynamic well and it needs a lot of energy – and green hydrogen – to get it back to those feedstocks we need to go on making things that we want in a way that keep the cost down.
What next?
So, we have a very large problem, some options – each of which has advantages and “issues”, and it will take a long time to change our supply chains, so we need to act fairly quickly before our filling the atmosphere with carbon dioxide does irreparable damage to our environment. We need a plan…
Written by David Bott, Director of Innovation at SCI and originally published on Linkedin
Batteries are a critical enabler for reaching net zero. As their importance increases, so does the need to better understand how they operate.
Mel Loveridge, Associate Professor (Reader) at Warwick University, gives an overview of the complexities of battery science and how she is working to bring increased understanding to a wider audience.
As the role of batteries has an increasing presence in everyday life, there is now a focus on battery forensic science and advanced characterisation methods – a critical part of understanding the life of a battery, its safety aspects and its cycle life or lifespan.
This forensic analysis and advanced characterisation is the core part the work carried out by Associate Professor (Reader) Mel Loveridge at Warwick University, who says: ‘The aim is to firstly understand and identify early-stage signatures of battery degradation, and ultimately to unearth the root causes and propagation of failure in lithium-ion battery (LIB) components.’
Since LIBs were commercialised in 1991, the electronic devices that use LIBs have diverged considerably, with much larger format batteries now required to electrify transport. This is a critical enabler that is needed if the world is to reach net zero.
‘Much research is focused on developing materials with higher energy and power density to effectively do this, and this is why battery safety considerations are more paramount now than ever,’ says Loveridge.
‘It is only by understanding how materials (electrodes and electrolyte) degrade using sophisticated forensic techniques, that we can feedback into the design of better, safer, more robust and stable components that will last longer,’ she adds.
This is key for the continued range and power improvements in electric vehicles, where ultimately everyday users will benefit from advances in battery materials and manufacturing processes.
Developing characterisation capabilities
This understanding requires effective characterisation capabilities to look at the chemical and structural dynamics that occur inside the battery as it ages. This can be accomplished destructively by autopsy when the battery has reached the end of its life (ex-situ) or done in real time whilst the battery is going through charge-discharge cycling (operando).
Because of the small size of the lithium atom, specialised X-ray based microscopy and other techniques are required to detect and map it. Fully understanding the complex journey of the lithium ions during battery operation is still challenging for the battery community.
Pictured above: A cathode particle. Copyright WMG
To facilitate this greater understanding, WMG was recently awarded an equipment grant to build the UK’s first multi-modal microscope platform with a plasma focused ion beam sectioning device (deliberately designed with batteries in mind, unlike other systems in existence). This includes a time-of-flight mass spectrometer to enable 3D detection and mapping of lithium. The integrated analytical platform will allow us to understand micro to meso scale structure and chemical dynamics over broad length and time scales.
The recent EU 2030 roadmap (Battery 2030+) stated “The accelerated discovery of stabilised battery materials requires special attention to the complex reactions taking place at the many interfaces within them.” Also awarded was a Lord Bhattacharyya PhD project to work on the commissioning and further development of this characterisation platform.
Breaking down the complexity for government and media
The work is highly challenging and riddled with complexities, but it has attracted significant media and government interest in the last decade and Loveridge has been one of the voices providing accessible, expert insight on a range of media platforms.
‘I have been fortunate to be interviewed for BBC2, Channel 4 and BBC Radio 4, describing how batteries work. I have also participated in energy-related panel discussions with the House of Lord’s Science & Technology Committee and the House of Commons Shadow Cabinet. Prior to this, an article I published on the temperature implications of wireless charging for a mobile phone battery was summarised in a feature in The Telegraph.’
The important work being carried out in battery forensic analysis is set to shape the future of battery technology.
Accelerating the transition to a sustainable global energy system
Welcome to the first in this series from the SCI Energy Group – we’ll be blogging regularly on topics of broad interest across the energy spectrum.

Andy Walker, Chair of the SCI Energy Group.
I’m Andy Walker, and I have the privilege of chairing the Energy Group, which comprises members drawn from industry, research institutes, universities, energy policy bodies, R&D organisations and scientific publishers. We meet regularly to discuss and organise events around the changing energy landscape, exploring challenges and opportunities associated with the clean energy transition.
We inform and influence climate change dialogue and policy in the UK and further afield, by taking a fact-based approach to the challenges and potential solutions, with the ultimate aim of making the global energy system sustainable. We do this by bringing together experts, influencers and other interested parties from across the technology, social science and policy landscape within industry, academia and government. In this way, the SCI Energy Group offers thought leadership, insight and debate around the clean energy transition.
Recently, the Energy Group Committee visited Imperial College London and were given a fascinating tour of the carbon capture and storage pilot plant, which Committee member Alex Bowles had very kindly organised. This was a really interesting visit, hosted by Dr Colin Hale and several enthusiastic and knowledgeable chemical engineering students, focused on the critical role that the capture and long-term storage (and utilisation) of CO2 will play within the clean energy transition. We learned that carbon capture utilisation and storage (CCUS) can play four critical roles in the transition to net zero:
- Tackling emissions from existing energy assets, for example by retrofitting existing fossil fuel-based power and industrial plants, by capturing the CO2 emissions emitted during these processes.
- As a solution for sectors where emissions are hard to abate, such as the in-process emissions during cement manufacture (one of the largest industrial sources of CO2 today).
- As a platform for clean hydrogen production – almost all of the 90 million tonnes of hydrogen generated today is via methane steam reforming, which emits around 10 tonnes of CO2 for every tonne of hydrogen produced.
- Removing CO2 from the atmosphere to balance emissions that cannot be directly abated or avoided (so-called direct air capture, DAC).
The International Energy Agency (IEA) estimates that the amount of CO2 captured and stored annually in their Sustainable Development Scenario rises to around 9.5 Gt per year by 2070, with another 0.9 Gt CO2 captured and used to make, for example, fuels and chemicals. (Note that a Gigatonne (Gt) is one billion metric tonnes).
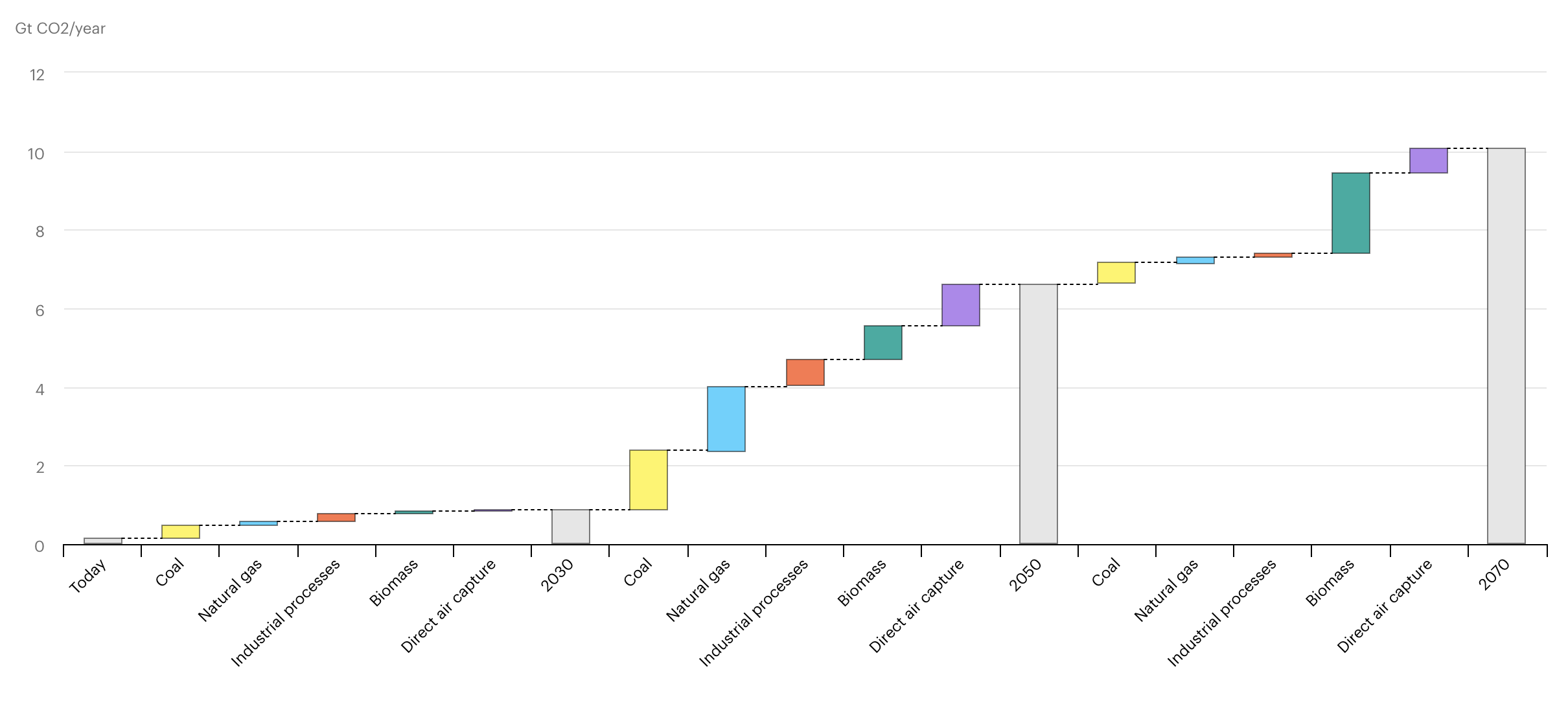
IEA, Growth in world CO2 capture by source and period in the Sustainable Development Scenario, 2020-2070, IEA, Paris. Licence: CC BY 4.0
The Energy Group plans to visit several other sites of interest in the coming months, including Drax and the Energy Innovation Centre in Birmingham, so look out for updates from these future visits.
Our next blog will relate to a recent workshop on Energy Storage, which we organised with strong support from Innovate UK/Knowledge Transfer Network. We brought in representatives from industry, academia, government and the finance sector to discuss this broad topic and to identify the key challenges, as well as outline some key policy questions for the government.
We chose this topic because energy storage is a critical part of the clean energy transition, as the world moves towards an increasing dependency on renewable sources of energy, which are inherently intermittent, yet it doesn’t receive enough attention and support from governments around the world. We’re sure you’ll find the outputs from this workshop very interesting!
Creating a paper pulp bottle that holds different liquids was a challenge that led BASF to join forces with Pulpex. Using sustainable chemistry the partners came up with an award-winning formula.
-
Vikki Callaghan, Packaging Project Manager, BASF plc
-
Tony Heslop, Senior Sustainability Manager, BASF plc
-
Scott Winston, CEO Pulpex Ltd
Could you start by explaining how the collaboration and the idea for the product came about?
Vikki: We had an existing relationship with Diageo. BASF and The Innovation Team at Diageo had worked on other projects addressing packaging needs. When the team had this idea for an innovative packaging solution they came to us. The challenge put to us was ‘Do you have the chemistry that will hold many different liquids in a paper pulp bottle?’ I love a challenge and was excited to get talking.
Scott: Having worked with BASF before, they were our natural choice to explore this conundrum. Diageo had the idea and an early proof-of-concept of a paper bottle, but it wasn’t utilising sustainable chemistry. The intellectual property was in place but the transformation of scientific proof-of-principle to scaled commercialised technology wasn’t something that could be done alone. The partnership with BASF naturally continued into Pulpex as it formed and continued to grow, remarkably, throughout the Covid-19 lockdown. BASF’s corporate purpose to create chemistry for a sustainable future was intrinsically aligned to meet our need to deliver a commercialisable product that could be produced at scale.
Tony: Following that first call in November 2019, we got together a couple of weeks later and enjoyed an intense deep dive workshop. This was going to take some time but if successful we knew this could be an impactful innovation. We set to work!

Testing out their bottle in the lab. Image courtesy of Pulpex Ltd.
What hurdles did you overcome in the development of the material?
Tony: The obvious hurdle was the pandemic. There were two years between our first and second face-to-face meeting. My initial thought was how do you drive an innovation process when you can’t get together. Surely constructive and productive collaboration isn’t possible? In fact, the inability to travel meant that we could talk more frequently despite our different geographical locations. Once we’d set up weekly online meetings, which evolved into smaller specialist break out groups, the process actually had many positives and the relationships, as well as the innovation, flourished.
Vikki: Of course, as with any innovation, we experienced technical challenges, too. There was no overall solution because we were looking at very diverse requirements and specifications. Different brand owners with different liquids meant there were many considerations and customised solutions required.
Sustainable packaging is a growing market with new products being launched. Can you explain where your product fits in and how is it different from similar materials?
Scott: Pulpex recognises the need to balance three critical aspects. Firstly, new packaging must continue to deliver established brand equity and meet consumer expectations on quality; secondly, any new packaging must technically deliver on performance through the supply chain starting with filling infrastructure compatibility and through distribution and critically, at end-of-life the packaging must be recyclable in existing infrastructure from collection to enable circularity, or where it does unfortunately escape to the environment, it must degrade and not leave an unintended legacy.
Vikki: The resulting fibre bottle is lightweight and offers brand owners a sustainable, environmentally-friendly alternative to plastic and glass bottles.

The final product. Image courtesy of Pulpex Ltd.
What are the main markets for the packaging? Are you able to comment on customers already using your product?
Tony: The innovation will be aimed at brand owners who want to have an alternative sustainable type of packaging, a product that is suitable for ‘on the go’ and that is easily recyclable through existing waste streams. The technology will hold a range of liquids from alcohol and detergent to shower gel, ketchup and engine oil.
Scott: Trials of the finished product have already started to take place with the most recent being at a corporate five-a-side football tournament at Wrexham AFC in May, where several hundred bottles were put to the test working with Severn Dee Water. Branded especially for the event and designed as a keepsake, the feedback from the public was resoundingly positive and it was great to see our bottles in action supporting those on the pitch.
What are the next steps for the BASF/Pulpex collaboration?
Scott: Having developed such a sustainable alternative packaging, our continuing sprint is scaling up! The technology has been developed and we are expecting to have bottles on shelves soon.
Vikki: We will have our ongoing quest of looking to hold a vast range of liquids and for different brand owners. We will have customised solutions, in different sizes, different shapes… the innovation and collaboration continues.

BASF and Pulpex won the SCI Innovation Enabled by Partnership Award 2023. Image credit: Andrew Lunn Photography
Links to previously published articles and videos (BASF/Pulpex/SCI)
- BASF May 2023 – SCI Award
- BASF August 2021 – Collaboration with Pulpex
- SCI News May 2023 – SCI Award
- Pulpex May 2023 – SCI Award
- Pulpex video
CCU International will supply its carbon capture and refinement system to Flue2Chem – a project led by SCI and Unilever which aims to convert industrial waste gases to create more sustainable consumer products. We caught up with CCU International CEO, Beena Sharma, to talk about her career path, motivations and challenges.
Tell us about your career path to date
I joined the Oil & Gas industry after university and began my career as a behavioural safety specialist, specifically for the construction phase of oil and gas projects. Soon after I joined the industry, I was assigned to an LNG plant in Nigeria for training and experience and eventually ended up at a gas plant in Norway before I returned to the UK. With both a psychology and training background I found myself working within a health, safety and environmental remit for various industries including healthcare, construction, manufacturing, and even the tobacco industry.

Beena and colleague at a gas plant in Norway, 2004. Image credit: Beena Sharma
What made you want to work in science and the environmental technology sector in particular?
When I moved to Scotland six years ago it gave me the opportunity to explore the ‘E’ in Health, Safety and Environment further, which was an area that I was always interested in but rarely got the attention it deserved in the industries I worked in. I volunteered on a Scottish climate change project, and this led me to think more deeply about the scientific and technological advances that were needed to achieve net zero by 2045 in Scotland. I knew this was a huge challenge with education, and changes in habit alone could not solve it.
I began to research solutions for hard-to-abate industries and areas that were a challenge to decarbonise, and set up my first business focused on a novel approach to insulating legacy buildings. I then worked on setting up a group of companies that included a solar PV installation company as well as a cleantech business that utilised an electrolysis technology to ozonate tap water for disinfection.
I was invited by my now business partner to help launch a biotechnical business that could create a circular food economy, taking food waste and creating microalgae for use in industries such as cosmetics, pharmaceuticals, and animal feed. This business incorporated 4 technologies, one of which was carbon capture. After some discussion with potential investors, it became clear that there was a huge interest and demand for carbon capture solutions. This led the team to decide to spin out CCU International as a separate entity and speed up the commercialisation of the technology which had been in development at the University of Sheffield under the lead of Peter Styring, Professor of Chemical Engineering and Chemistry.
Which aspects of your work motivate you the most?
The aspects of what I do that motivates me the most is the educational role that I play as the CEO of the business. I am regularly invited to speak on panels, podcasts, webinars and at conferences to share my knowledge with an industry that is transitioning and eager to learn, grow and incorporate new ways of thinking and doing things. It is extremely rewarding to see that people have come away from listening to me with a new perspective and being inspired to go away, take that learning, incorporate it in their ways of working and become innovators themselves.
According to the UN, carbon capture will be a key technology in achieving net zero. It is extremely rewarding to know that the CCU International technology will be a major contributor to this goal and that we can enable decarbonisation with the technology usage across multiple industries, both large and small, which otherwise would not have been possible.
What have been the biggest challenges for you as an entrepreneur?
As an entrepreneur my biggest challenge has been establishing myself in an industry and environment that is not well represented by women, and in particular women of colour. Often, it comes as a surprise to many that I would be heading up such a business and unfortunately many biases still exist within all genders and backgrounds. It makes it that extra bit harder and there can be a requirement to prove oneself as credible through knowledge or capability before the respect is given.

Image credit: Beena Sharma
The other big challenge has been around the education we provide for all our stakeholders. Innovation is not always welcome, especially in an industry or area where it may seem innovation is not needed. As the saying goes, ‘if it’s not broke, don’t fix it’, so stakeholders tend not to realise there is a problem until we educate them on the solution! And not many can accept there may be a better way of doing things than what they themselves have been doing for years!
What would be your top piece of advice for anyone thinking of starting up their own SME?
Starting up in business is a step that many think about doing but very few actually do. Most would be led to believe that you would need to work for months, maybe years on market research, business planning, strategy etc. before starting a business. My one piece of advice would be to start. Most of what you learn will come from doing. It is essential for entrepreneurs to fail, make the mistakes and learn what not to do next time so you have a better chance of success going forward. Many successful businesses emerge from failure.
What is it about the Flue2Chem project that is unique, what made you want to get involved, and what is the potential difference this project could make?
The Flue2Chem project is aimed at converting industrial waste gases into sustainable materials for use in consumer products. What is unique about the Flue2Chem project is that organisations that would normally be competitors have come together to find a solution for a problem that affects us all – as people, as businesses and as a planet. It is rare to see such cross-industry collaboration on this level and this allows both cross-learning and inspires others to come together, collaborate and innovate to solve problems that affect us all, much like the Flue2Chem project. It is a privilege to be part of the project by contributing our technology to the capture component.

CCU International, carbon capture technology. Image credit: Beena Sharma
The project will play a key role in supporting the UK’s 2050 net zero ambitions by providing a more sustainable feedstock for products such as household cleaning materials. The project could demonstrate how the UK could cut 15-20 million tonnes of carbon dioxide emission each year. The UK imports large quantities of carbon containing feedstocks that we use in the consumer goods industry. The project will demonstrate how we can secure an alternative domestic source of carbon for these goods and also demonstrate how industry can contribute towards achieving net zero.
Why do you think collaboration of this scale is so important?
Industry coming together to solve climate change issues is essential if we are ever to achieve net zero. Collaboration of this scale sends a strong message and emphasises that change in approach is needed and that innovation is key. This inspires others to do the same. Solutions are needed now and by bringing expertise and experience together we learn and adapt quicker. Solutions are needed now – not in years to come.
The impact this project will have has the potential to be huge, across multiple industries and certainly with how we look at not only capturing carbon emissions but also what we can do with the captured carbon dioxide, promoting a circular carbon economy where in time we learn to value carbon dioxide in a way that has never been done before.
Certainly, for the carbon capture storage community, this project will show that there is a use for captured carbon dioxide other than treating it as a waste and sequestering in underground oil reservoirs. Utilising captured carbon dioxide can create revenue streams for any business or process that emits carbon dioxide.
The collaboration demonstrates the commitment from industries to support decarbonisation, of those industries that are hard to abate whilst at the same time building a new UK value chain.
Rarely have science and government been as clearly linked as the initial response to the Covid-19 pandemic, when politicians could be heard claiming they were being ‘led by the science’ as often as they could be seen doing that pointing-with-a-thumb-and-fist thing.
This Thursday, the UK’s Chief Scientific Adviser, Sir Patrick Vallance, will receive the Lister Medal for his leadership during the Covid-19 pandemic, and you can stream it live here, exclusively on SCI’s YouTube channel!
In readiness for Sir Patrick’s lecture, Eoin Redahan looks back at three ways science helped to mitigate the spread of Covid-19.
People will never look at vaccine development the same way. For good or ill, we have realised just how quickly they can now be developed. Similarly, we have realised what can be achieved when the brightest brains come together. These are two of the positive legacies from Covid.
But there are others. Some of the innovations conceived to tackle Covid will now tackle other pathogens. Here are just three of the innovations that emerged…
1. Wastewater warning

As Oscar Wilde once said: ‘We are all in the gutter, but some of us are looking up at the genetic material in stool samples.’
Not many people would find inspiration in wastewater treatment plants when thinking about early warning systems for infectious diseases.
Nevertheless, during the Covid-19 pandemic, researchers at TU Darmstadt in Germany came up with a system that detected Covid infection rates in the general population by analysing their waste – a system so accurate they could detect the presence of Covid among those without recognisable symptoms.
To do this, they examined the genetic material in samples from Frankfurt’s wastewater plants and tested them using the PCR test. They claim that their measurement was so sensitive it could detect fewer than 10 confirmed Covid-19 cases per 100,000 people.
It is inevitable that Covid-19 variants will rise again, but this system could alert us to the need for tighter protective measures as soon as the virus appears in our wastewater.
2. UV air treatment
UV light can reportedly reduce indoor airborne microbes by 98%.
Warning systems are important, as are ways to stop the spread of pathogens. To do this, a team from the UK and US shed light on the problem – well, they used ultraviolet light to remove the pathogens.
Using funding from the UK Health Security Agency, Columbia University researchers discovered that far-UVC light from lights installed in the ceiling almost eliminate the indoor transmission of airborne diseases such as Covid-19 and influenza.
The researchers claim it took less than five minutes for their germicidal UV light to reduce indoor airborne microbe levels by more than 98% – and it does the job as long as the light remains switched on.
‘Far-UVC rapidly reduces the amount of active microbes in the indoor air to almost zero, making indoor air essentially as safe as outdoor air,’ said study co-author David Brenner, director of the Center for Radiological Research at Columbia University Vagelos College of Physicians and Surgeons. ‘Using this technology in locations where people gather together indoors could prevent the next potential pandemic.’
3. Biological masks?

‘Physical mask, meet biological mask.’
Many moons ago, it was strange to see a person wearing a mask, even in cities with dubious air quality. Now, they are ubiquitous, and it would appear that mask innovations are everywhere too.
During Covid, researchers from the University of Granada in Spain were aware that wearing masks for a long time could be bad for our health. They devised a near field communication tag for inside our FFP2 masks to monitor CO2 rebreathing. This batteryless, opto-chemical sensor communicates with the wearer’s phone, telling them when CO2 levels are too high.
In the same spirit, researchers in Helsinki, Finland, developed a ‘biological mask’ to counteract Covid-19. The University of Helsinki researchers developed a nasal spray with molecule (TRiSb92) that deactivates the coronavirus spike protein and provides short-term protection against the virus – a sort of biological mask, albeit without those annoying elastics digging into our ears.
‘In animal models, nasally administered TriSb92 offered protection against infection in an exposure situation where all unprotected mice were infected,’ said Anna Mäkelä, postdoctoral researcher and study co-author.
‘Targeting this inhibitory effect of the TriSb92 molecule to a site of the coronavirus spike protein common to all variants of the virus makes it possible to effectively inhibit the ability of all known variants.’
The idea is for this nasal spray to complement vaccines, though during peak Covid paranoia, it might be tricky persuading everyone on the bus that you’re wearing a biological mask.
Covid disrupted scientific progress for many, but as we know, invention shines through in troublesome times. Plenty of innovations such as the ones above will make us better equipped to tackle air borne diseases – alongside the stewardship of leaders like Sir Patrick Vallance.
Watch Sir Patrick Vallance’s talk – Government, Science and Industry: from Covid to Climate – at 18:25 on 24 November
What does clean smell like? What if the fragrance you want to create is that of a sweet-smelling, yet poisonous, flower? In his Scientific Artistry of Fragrances SCITalk, Dr Ellwood led us by the nose.
When Dr Simon Ellwood spoke about creating a fragrance, it sounded like a musical composition or a painting. The flavourist, sitting before a palette of 1,500 fragrance ingredients. Each occupies a different note on the register: the top notes, the middle ones, and the bottom.
To the outsider, this seems impossibly vast and daunting. The Head of Health & Wellbeing Centre of Excellence – Fragrance and Active Beauty Division at Givaudan mentioned that Persil resolved to come up with ‘the smell of clean’ for its detergents in the late 1950s.
But what should clean smell like? Should it be the green, citrusy aromas of this laundry detergent, the smell of mint, or the antiseptic at the hospital?
To make choosing smells slightly less daunting for flavourists and perfumers, they are at least split into odour families such as citrus, floral, green, fruity, spicy, musky, and woody. Some of these ingredients are natural, some are inspired by nature, and others come from petrochemicals and synthetic materials.

The delicious-smelling musk deer.
Deer gland perfume
One of the smells you may have sprayed on your person – one sibling in this odour family – has peculiar origins. The pleasant, powdery smell known as musk was originally extracted from the caudal gland of the male musk deer and from the civet cat.
But as the Colognoisseur website notes, as many as 50 musk deer would have to be killed to obtain one kilogramme of these nodules. Now, killing a load of deer and cats for a few bottles of perfume may not have seemed unethical several centuries ago, but it also wasn’t sustainable or cost-effective. It became clear that a synthetic musk was needed.
When the synthetic musk discovery came in 1888, it was a chance discovery. Albert Bauer had been looking to make explosives when a distinctive smell came instead, along with the scent of opportunity.
>> Read about the science behind your cosmetics

Dior recreated the woodland notes of Lily of the valley.
Do you like the smell of jasmine?
Dr Ellwood’s talk laid bare not only the vastness of everything we smell, but also the ingenuity of those who recreate these odours. In terms of breadth of smell, neroli oil – which is taken from the blossom of a bitter orange – has floral, citrus, fresh, and sweet odours, with notes of mint and caraway. Similarly, and yet dissimilarly, jasmine’s odour families are broken down into sweet, floral, fresh, and fruity, and – jarringly – intensely fecal.
The ingenuity of flavourists is exemplified by lily of the valley. The woodland, bell-shaped flowers are known for their evocative smell, but all parts of the plant are poisonous. Despite this, French company Dior synthetically recreated the lily of the valley smell in its Diorissimo perfume in 1956 using hydroxycitronellal, which is described by the Good Scents Company as having ‘a sweet floral odour with citrus and melon undertones’.

Cyanide smells like almonds, but you might not want to eat it.
Of course, as Dr Ellwood noted, synthetic flavours can only ever get so close to the real thing – an imperfect facsimile. However, the mere fact that chemists have recreated deer musk, lily of the valley, and the prized ambergris from sperm whales to create the fragrances we love is almost as extraordinary as the smells themselves.
‘Fragrance,’ he said, ‘will always be the confluence of the artistry of the perfumer and the chemist.
Register for our free upcoming SCI Talk on the Chemistry behind Beauty & Personal Care Products.
Do you know how the Academy Awards came to be named the Oscars? What about the story behind the Nobel prize? Behind every award name there is a story, and the Julia Levy Award is no exception.
On the face of it, the Julia Levy Award is about innovation in biomedical applications, but it is the stories of the winners of this SCI Canada award, and Julia Levy herself, that really give it life.
But for a tweak of history, Julia Levy may not have ended up in Canada at all. Born Julia Coppens in Singapore in 1934, she moved to Indonesia in her early childhood. Her father uprooted the family during the Second World War and she left for Vancouver with her mother and sister – her father only joining them after release from a Japanese prisoner-of-war camp.
Julia and her family moved to Vancouver during the Second World War.
After studying bacteriology and immunology at the University of British Columbia (UBC), the young Julia received a PhD in experimental pathology from the University of London. She went on to become a professor at UBC and helped found biopharmaceutical company Quadra Logic Technologies in 1984.
More important than confining her achievements in cold prose, Julia Levy’s work made a profound difference to people’s lives. She developed a groundbreaking photodynamic therapy (PDT) that treated age-related macular degeneration – one of the leading causes of blindness in the elderly. She also created a bladder cancer drug called Photofrin in 1993 and, according to Neil and Susan Bressler, the Visudyne PDT treatment created by Julia and her colleagues was the only proven treatment for certain lesions.
Levy thrived in the business space too, serving as Chief Executive Officer and President of QLT from 1995 to 2001. She has since won a boatload of awards for her achievements, but sometimes the best testimonies come from those who have been inspired by her achievements.
Trailblazing drug discovery systems
For Helen Burt, winner of the 2022 Julia Levy Award and retired Angiotech Professor of Drug Delivery at the University of British Columbia (UBC), Julia has been an inspiration. Here was this UBC professor who jointly founded this big, exciting company – creating medication that improved people’s lives and showing her what was possible.
Helen, an English native, moved to Vancouver in 1976 for her PhD and loved it so much that she stayed. As a professor at UBC, Helen would become a trailblazer in drug delivery systems – a field pioneered earlier by Julia Levy.
‘I was a new assistant professor when she was building Quadra Logic and I would go to talks that she gave,’ Helen said. ‘Essentially, the early technology for QLT was a form of very sophisticated drug delivery [...] It was getting the drug they developed into the eye and irradiating it with light of a specific wavelength.
‘It was very, very targeted. And so, you didn’t get the drug going elsewhere in the body and causing unwanted side effects. So her technology was a form of very advanced drug delivery technology.’

‘For me to win an award that honours Julia Levy and her achievements – I think that's what makes it so special to me.’ – Professor Helen Burt, a former student of Julia Levy, is the Award's most recent recipient.
>> Learn more about SCI Canada.
These talks chimed with the young Helen. If a microbiologist could develop this kind of technology, what was stopping her from developing her own?
She, too, became a pioneer in her field, developing nanoparticle-based drug delivery systems (including those to treat cancer) and a novel drug-eluting coronary stent. According to Professor Laurel Schafer, who put Helen forward for the Julia Levy Award: ‘[Helen] was a trailblazer in new approaches for drug delivery and in research leadership on our campus.’
Importance to Canadian chemistry
Professor Schafer is a hugely accomplished chemist in her own right; and the University of British Columbia chemistry professor’s achievements in catalysis discovery were recognised with the LeSueur Memorial Award at the 2020 Canada Awards.
Julia Levy provided an inspiration to Laurel too, in her case as an exemplar for what Canadian chemists could achieve. ‘The achievements of Julia Levy show that it really can be done right here in Canada, and even right here in British Columbia,’ she said. ‘I grew up in a Canada where I believed that better was elsewhere and our job was to attract better here – a very colonial attitude.

Julia studied at and later became a Professor at the University of British Columbia – the campus is pictured above.
‘I now believe and know that better is right here. Professor Levy’s work showed that world-leading contributions come from UBC and from the laboratories led by women.’
She noted that the Julia Levy Award acknowledges Canadian innovation in health science, whereas Canadian chemistry has historically focused on process chemistry in areas such as mining and petrochemicals.
But Julia Levy’s influence permeates beyond science. ‘Julia is one of those people who has been willing throughout her whole career – even now, well into her eighties – to give back to the community,’ Professor Burt says. ‘She mentors, she coaches, she sits on the boards of startup companies, and she advises.’
‘She’s just got this incredible amount of knowledge… She was the Chief Executive Officer [at QLT], so she learnt all of the aspects: the complex and sophisticated regulations, knowing how to find the right people to conduct clinical trials, and how to do the scale-up. She really is a legend in terms of giving back to the community. And this is not just in British Columbia – it’s Pan-Canadian.’

Pictured above: Julia Levy
For young chemists, the Julia Levy in the Julia Levy Award may just be a name for now, but for those in the Canadian chemical industry and patients all over the world, her influence and her work resonate.
As Professor Helen Burt said: ‘For me to win an award that honours Julia Levy and her achievements – I think that's what makes it so special to me.’
>> For more information on the Canada Awards, go to: https://bit.ly/3VMwNKa
From government grants to analysing your own carbon footprint, energy-efficient measures could reduce the environmental impact of your SME and save you money. Retail Merchant Services explained some of the changes you could make.
Which measures could Small and Medium-sized Enterprises (SMEs), especially energy-intensive businesses, take right now to reduce their carbon footprints?
- Look at the sustainability of the products you are creating. Can you use renewable materials? Or, if you get materials from another supplier, can you check their eco-credentials, and swap if they’re not doing what they can to go greener? Can you streamline the process so that you reduce waste wherever possible?
- Create a recycling policy. If you can’t reduce the amount of waste that you’re generating, you should aim to make sure that it can be disposed of sustainably. You could look at your shipping supply chain. If your business sends out physical items, make sure to check the packaging you’re using – can it be recycled? Are you using too much?
- Can you switch to a renewable energy supplier, or generate your own renewable energy? While it’s not right for everyone, it can also be worth considering if there are any employees in your business who can work from home on some days. This removes the carbon footprint of their journey to work.
The Smart Export Guarantee Scheme pays some SMEs for producing their own renewable heat and power. Not only will this allow you to generate your own electricity, which can be useful in the current climate of fluctuating costs, but you can earn money from this too.
The Clean Heat Grant is a government-backed grant that rewards companies who use green heating technologies like heat pumps, and the Green Gas Support Scheme is intended to increase the amount of green gas in the National Grid.
The amount that SMEs can benefit from these schemes may depend on the amount of money that they have available to buy renewable technology, or the space to put items like heat pumps. If this is likely to be a barrier, then they may find smaller local schemes more useful.
Do you have any tips for companies calculating their carbon footprints? What are the potential benefits of this?
Take your time – understanding your carbon footprint isn’t an overnight process. You may find it beneficial to use an online carbon footprint calculator, or contact a sustainability expert to help.
You’ll need to consider three types of emissions:
- Direct emissions – the emissions that your company is directly responsible for, such as fuel for company cars.
- Energy indirect emissions – emissions from utilities that you don’t directly control, such as electricity and gas
- Other indirect emissions – everything else that is connected to your business activities, such as employee travel, shipping, and the whole supply chain.
Understanding your carbon footprint is important to help you know where you can improve and cut down on your emissions. Not only does this help the planet, but it’s also a tangible demonstration that you care about the environment, which can be attractive to sustainably-minded customers.
The initial outlay for heat pumps and other technologies are steep, but this investment may pay off in the longer term.
What are the benefits of aggressively pursuing net zero and what are the drawbacks?
Of course, the primary benefit of pursuing net zero is that it helps the planet. Business waste has a huge impact on the environment and, as a result, any changes that can be made in this sector will have a big impact too. However, going net zero can also potentially make your business more profitable too.
Your profits may go up for several reasons. First, it’s more appealing to customers. As part of going net zero, you’re likely to adjust your products to be more eco-friendly. And with reports showing that 63% of millennials are willing to pay more for sustainable products, this could make your business more appealing.
Second, it could save you money. You may find that examining your processes and policies to make them greener will allow you to benefit from specific tax cuts, or simply improve the efficiency of your company. In time, this could save you from wasting money as well as energy.
Third, it could boost your competitiveness. Small companies often find they just can’t match big businesses for price, so it’s important to find a selling point that allows you to remain competitive. As mentioned before, customers are increasingly looking for more ethical products, so being able to say that you’re net zero could help you beat the competition.
Finally, it could prepare you for new policies. Governments around the world are under pressure to go greener, and so they’ll likely transfer this pressure to businesses. Going green now means you’ll be ahead of the curve and able to make these changes at your own pace, rather than having to rush and pay to make them all at once.
While these are all amazing benefits, one of the biggest challenges that SMEs face is the cost of going net zero. It’s not cheap in the current economic climate, especially if you’ve got big changes to make. According to research, 40% of SMEs said that high cost and lack of budget were the biggest net zero blockers.

Electric vehicles require less maintenance – and you don’t have to pay road tax.
What are the benefits of moving your vehicles to electric right now, and what are the drawbacks?
There’s no denying that electric vehicles are significantly better for the environment than conventional cars. For companies looking for a relatively straightforward way to go greener, electric cars can be a great choice.
As well as swerving rising fuel prices, EV owners don’t currently pay vehicle tax in the UK. Additionally, they have fewer moving parts, and so require less maintenance. All of these factors mean that while EVs can be an expensive initial investment, they generally cost less to run in the long term.
With the UK government banning new petrol and diesel vehicles from 2030, investing in electric vehicles now means that SMEs can get ahead of the rush that is likely to come as we get close to the deadline. There is already a year’s wait time for some vehicles, so ordering your fleet now could mean that you avoid an even longer queue further down the line.
Of course, many SMEs feel unable to commit to electric vehicles right now due to the cost of living – they’re an expensive purchase. If this is the case, you could consider changing one vehicle at a time, and looking to see if you’re eligible for any local grants that can support you with the cost of this.
How much have inflated energy costs undermined the push for net zero?
Unfortunately, rising energy costs have meant that small businesses are feeling the pinch, and might struggle to make new eco-friendly changes, as they are often costly. For many, their focus is simply remaining profitable.
However, what is also clear is that for those that can afford it, examining your business for changes that will allow you to move towards net zero can also be a way of saving money in the long run.
If you’re able to produce your own renewable energy (for example, getting solar panels on the offices), you may be able to mitigate some of the effects of rising energy costs, as you won’t be reliant on the National Grid.
Finally, apart from energy efficiency schemes, how could the government help reduce the carbon footprint of SMEs?
As well as energy schemes, the government can help by providing information and resources on sustainable practices. By sharing best practices widely with businesses, and offering them a place to go to get support, the government can help them develop more environmentally friendly operations.
Additionally, they can help by creating incentives for businesses to go green. By offering tax breaks or other financial incentives, the government can encourage businesses to adopt sustainable practices.
Written by Retail Merchant Services. The SME Environmental Impact Guide can be read in full.
Edited by Eoin Redahan.
Little machines that blend makeup tailored for your skin alone… Technology that details the tiny creatures walking on your face… The cosmetic revolution is coming, and Dr Barbara Brockway told us all about it.
Max Huber burnt his face. The lab experiment left him scarred, and he couldn’t find a way to heal it. So, he turned to the sea. Inspired by the regenerative powers of seaweed, he conducted experiment after experiment – 6,000 in all – until he created his miracle broth in 1965. You might know this moisturiser as Crème de la Mer.
A rocket scientist in the world of cosmetics seems strange, but when you interrogate it, it isn’t strange at all. As Dr Barbara Brockway, a scientific advisor in cosmetics and personal care, explained in our latest SCItalk, cosmetics hang from the many branches of science.
Engineering, computer science, maths, biology, chemistry, statistics, artificial intelligence, and bioinformatics are among the disciplines that create the creams you knead into your face, the sprays that stun your hair in place. They say it takes a village to raise a child, and it takes an army of scientists to formulate all the creams, gels, lotions, body milks, and sprays in your cupboard.

Some say sea kelp can be used to treat everything from diabetes, cardiovascular diseases, and cancer, to repairing your nails and skin.
There is a reason why the chemistry behind these products is so advanced. If you sell bread, it is made to last a week. If you make a moisturising cream, it is formulated to last three years. To make sure it does that, chemists test it at elevated temperatures to speed up the time frame. They conduct vibration tests and freeze-thaw tests to measure its stability.
Dr Brockway likened the process of bringing a product to market to a game of snakes and ladders. If you climb enough ladders, you could take your own miracle brew to market within 10 months.
But expectations are high, and the product must delight the user. Think of the teenager who empties a half a can of Lynx Africa into his armpit, or the perfume that is a dream inhaled. Each smell she likened to a musical composition.
But these formulators are not struggling artists. Perfumers and cosmetic chemists – these bottlers of love and longing and loss – can earn a fortune. Dr Brockway’s quick calculation provided a glimpse of the lucre.
Take 15kg of the bulk cream you mixed on your kitchen table. That same cream could be turned into 1,000 15ml bottles, each sold for £78. So, just 15kg of product could fetch £78,000. So, it’s easy to see why the global beauty market is worth $483 billion (£427 billion), with the UK market alone worth £7.8 billion – more than the furniture industry.
Smart mirror, mirror, on the wall…
It’s unsurprising that an industry of such value and scientific breadth embraces the latest technologies, from those found in our phones to advances in genetics and the omics revolution.
Already, the digital world has left the makeup tester behind. Smart mirrors overlay virtual makeup, recommend products for your complexion, and even detect skin conditions. Small machines that look like coffee-makes blend bespoke makeup. Indeed, Dr Brockway noted that Yves Saint Laurent has created a blender that produces up to 15,000 different shades.
Even blockchain has elbowed into the act. It is being used to make sure that a product’s ingredients aren’t changed in between batches. By showing customers every time-stamped link of the supply chain, companies can prove that their products are organic or ethically sourced. The reason why blockchain is significant here is that, once recorded, the data stored cannot be amended.
At first glance, proving the provenance of materials to customers might seem like a marketing ploy, but this is also being done in response to the increasing fussiness of the consumer.
Collagen is the main component of connective tissue.
Dr Brockway said all brands are now under pressure to incorporate sustainability into their business practices. The younger age group is also looking for more organic, vegan-friendly ingredients, and businesses have had to respond.
For example, microbial fermentation is being used instead of roosters’ coxcombs to create hyaluronic acid. Similarly, Geltor claims to have created the first ever biodesigned vegan human collagen for skincare (HumaColl21®). Such collagen is usually provided by our friends the fish.
These advances are significant, certainly to the life expectancies of roosters and fish, but of such ingredients revolutions are not made. Other forces will shake the industry.
Meditating on omics
Back in the 1970s, scientists thought the microbes that live on our skin were simple, but next-generation DNA technology reveals that thousands of species of bacteria live on our skin (a pleasant thought). Dr Brockway says these microbes tell us about our lifestyles – to the point that they even know if you own a pet.
So, what is the significance of this? Developments in DNA technology and omics (various disciplines in biology including genomics, proteomics, metagenomics, and metabolomics) mean we can now get not just a snapshot, but an entire picture of what’s going on on your face.
‘Thanks to omics we really know what’s now going on with our skin and see what our products are doing,’ Dr Brockway said. ‘We know the target better. We know which collagens, out of the 263, we need to encourage.’
We are learning more and more about how our skin behaves. And those time-honoured potions and lotions espoused by our grandparents – it will make sense soon, not just why they work, but why they work for some and not for others. In cosmetics, we are leaving the era of checkers and entering the age of chess.
This is the first of three cosmetic SCItalks between now and Christmas. Register now for the Scientific artistry of fragrances.
Eye-catching infographics, punchy messaging, and clear language are just three ways to grab people’s attention. Laura West, Senior Scientific Excellence Coordinator of R&D Biopharm Discovery at GSK, explains how to make your scientific research more visually attractive.
When it comes to displaying your scientific work, the experiments and data could be your best, but getting the visibility your work deserves and engaging your target audience require careful thought. It is, therefore, vital to be to think about how you communicate, not just what you communicate.
Every day, we are inundated with information. It’s more important now than ever to grab the attention of your audience, while improving the way you communicate. This helps people retain information about the data and key messages you deliver.
Ask yourself: what is the key message I want people to take away from this piece of work? You can then start to build around that.
When it comes to the overall layout of your work, you need to think about visual hierarchy, which is the arrangement of the elements on the page. This tells readers what to focus on depending on its importance.
It’s also worth thinking about how people best consume their media. Infographics, data visualisation graphs, images, and short videos are all great ways to attract and hold people’s attention.
Here are five ways to boost engagement in your work today.
1. Start with a bold, catchy message

Image from Naja Bertolt Jensen, Data: Plastic Pollution - Our World in Data. Graphic from Laura West
A clear, simple message that is big, bright, bold and catchy will grab people’s attention. Take a look at the infographic below. Notice how your eyes are immediately drawn to ‘Plastic Pollution’, which is short, punchy, and immediately noticeable.
2. Pick relevant images

65% of people recall information for up to three days when it is paired with a relevant image. So, pick relatable images to make your work more memorable.
3. Keep it simple
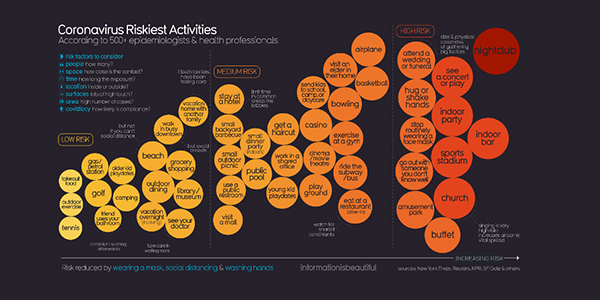
Covid 19 Infographic Datapack from Information is Beautiful.
Aim to keep your sentences short and use simplified language. This approach will make your work more accessible and easier to understand, and it will help your audience retain information.
Second, if you have a large amount of data, consider how to display it so that people can immediately follow what you’re showing them.
Take a look at the ‘Coronavirus Riskiest Activities’ infographic below. You can immediately see that ‘nightclub’ is the riskiest activity from the huge amount of information on the page. Note the use of negative space (or empty space) on the page to intensify the size of each bubble.
4. Use colour and contrast
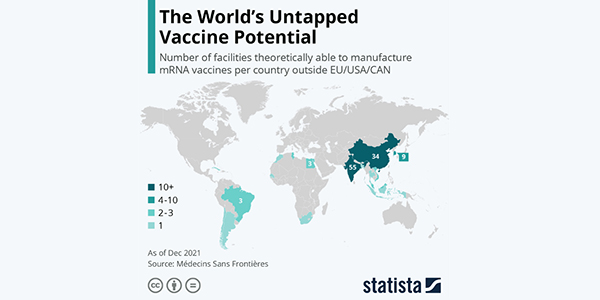
This infographic from Statista uses a simple colour scale to clearly demonstrate the data.
Colour choice matters. Our eyes pick up the contrast between certain colours and using this to your advantage will help accentuate the importance of certain items on the page. Think about the contrast between the colours you are displaying to make the text or imagery striking. This helps readers associate patterns or trends quickly.
In the image above, for example, it is easy to identify the teal colours against the white background and grey world map and immediately identify the countries.
5. Think about how people read
Readers use a 'Z' pattern to visually skim content.
Studies show that when we ingest digital information, we first scan the page in a ‘Z’ or ‘F’ pattern to determine whether it is worth reading.
If the information is predominantly text heavy, we read by scanning the left side of the page as this contains left aligned headings and bullet points. When reading information that is not in text-heavy paragraphs, we tend to read in the more ‘Z’ aligned format (left to right and top to bottom).
When thinking about the type of work you are displaying, consider where you want your most important information on the page.
Reading outside his research area and efficient chemistry helped 2022 Perkin Medal winner Dennis Liotta develop groundbreaking drugs.
There has been an explosion of statistics in football, but one of the most influential figures in this revolution, Ramm Mylvaganam, didn’t care for the game. He worked for the confectionary company Mars. He sold chairs. He knew nothing about football.
However, this key figure outlined in Rory Smith’s recent book, Expected Goals: The story of how data conquered football, came into the field of football analysis and changed the game forever – partly because he approached the game with the fresh perspective of the outsider.
So, what do football statistics have to do with a chemist who came up with life-saving medications? Well, Dr Dennis Liotta, who came up with AIDS antivirals that have saved thousands of lives, may not have entered medicinal chemistry as a complete outsider. He was a chemist, after all. However, like Ramm Mylvaganam, his broad breadth of knowledge from different areas gave him a unique perspective on a new field.
Reading at random
Dr Liotta didn’t take the standard path into medicinal chemistry. In fact, he wasn't a diligent chemistry student at first – and that, in an odd way, contributed to his later success.
For the first couple of years at university, he was more interested in his extracurricular activities; but in his third year, he realised he needed to catch up. He worked hard and burnt the midnight oil. He also did something unusual.
‘I did something that’s kind of ridiculous-sounding,’ he said. ‘I had this big fat organic chemistry book, and I would just open it up randomly to some page and read 10 or 12 pages and close it back up. Over time, I ended up covering not only the things I missed, but actually learning about a lot of things that wouldn't have been covered.’
As his career progressed, Dr Liotta realised the importance of not just working harder, but working smarter. On Sundays, he would sit down with a bunch of academic journals to stay abreast of developments. However, as he read them, he discovered other papers – ones outside his research area – that piqued his interest.

Dennis Liotta in one of his lab spaces at Emory. Image by Marcusrpolo.
‘I’d see something intriguing. And so I’d say, that’s interesting, let me read. I started learning about things that I didn’t technically need to know about, because they were outside of my immediate interest. But those things really changed my life. And, ultimately, I think they were the differentiating factor.’
The intellectual stretch
This intellectual curiosity led to more than 100 patents, including a groundbreaking drug in the fight against AIDS that is still used today and a hand in developing an important hepatitis C drug.
‘In science, many times the people who actually make the most significant innovations are the people who come at a problem that’s outside of their field,’ Dr Liotta said. ‘Without realising it, we all get programmed in terms of how we think about problems, what we accept as fact.’
‘But when you come at a problem that’s outside your field… you aren't immersed in it. So, you think about the problems differently. And many times, in thinking about the problems differently, you’ll come up with an alternative solution that people in the field wouldn’t.’
We’ve often heard the stories of Steve Jobs wandering into random classes while at university when he should have been attending his actual course. Apparently, a calligraphy class inspired the font later used in Apple’s products. In other words, early specialism can sometimes hinder creativity.
‘I've looked into people who have made really some amazing contributions, and many times there’s been an intellectual stretch,’ Dr Liotta said. ‘They’ve gone out there and done something that they weren’t really trained to do. You can fall on your face from time to time, but it’s really nice when we're able to make contributions in areas where we don’t really have any formal training.’
Chance favours…
Of course, there’s so much more to creating life-saving drugs than intellectual curiosity and a different way of thinking. Dr Liotta and his colleagues had the technical skill to turn their ideas into something real. He was a skilled chemist who teamed up with an excellent virologist, Raymond Schinazi. The result of this blend of their skills gave them an edge over others developing AIDS therapeutics.

Dr Liotta invented breakthrough HIV drug Emtricitabine.
‘The very first thing we did was we figured out a spectacular way of preparing the compounds – very clean, very efficient,’ he said. ‘And that [meant we could] explore all sorts of different permutations around the series of compounds that others couldn’t easily do, because their methods were so bad for making [them].
‘So, even though we were competing against some very important pharmaceutical companies that had infinitely more money than we had – dozens of really smart people they put on the project – we were able to run circles around them because we had a really efficient methodology and that enabled us to make some compounds.’
The amazing thing is that the very first compound and the third compound the pair came up with led to FDA-approved drugs. It is a fine thing, indeed, when skill and serendipity meet.
‘Chance favours the prepared mind,’ Dr Liotta said, ‘or, as my colleagues say: you work hard to put yourself in a position to get lucky.’
>> Learn more about Dr Liotta’s career path and research from our recent Q&A.
There is still work to be done to redress racial inequality in chemistry, and across science in general, but relatable role models can have a positive influence on the next generation.
Homophily. Ever heard of it? Me neither, until 30 minutes ago. Homophily basically means that we are more likely to connect with people who are similar to us in some way.
In work terms, homophily could be a relatable role model. So, as an Irish science writer, I admire Flann O’Brien for his ability to decongest complicated subjects with such wit and flair (not so much for hiding whiskey in the toilet during interviews). For a young chemist, a role model could be someone from a similar background who excels in a job she or he would love to have.
But what happens if you just don’t see relatable role models in your chosen field? What if systemic failings make the profession less attractive and harder to trace the path to success?
Unfortunately, systemic failings, the relative lack of homophily, and pervasive inequality were among the findings of Missing Elements – Racial and ethnic inequalities in the chemical sciences, a report released by the Royal Society of Chemistry (RSC) in March.
The report highlighted the barriers facing Black chemists in the UK, and it certainly didn’t hold back. In the Foreword, Dr Helen Pain, RSC’s Chief Executive, said: ‘The data and evidence collected in this report are clear: we are failing to retain and nurture talented Black chemists at every stage of their career path after undergraduate studies.’
The report found that just 1.4% of postgraduate students, 1% of non-academic chemistry staff, and 0% of chemistry professors are Black. It added that Black chemists face barriers in industry too, and that people from minoritised communities are under-represented at senior levels across the workforce.
It proceeded to mention six themes that affect the retention and progression for Black chemists, including the impact of homophily, which it defined as ‘the tendency for people to form connections with people similar to themselves.’
The importance of mentors
When I read that, a little bell chimed in my head. When my colleague Muriel Cozier interviewed three eminent Black chemists last year – Cláudio Lourenço, Jeraime Griffith, and Dr George Okafo – each mentioned the need for relatable role models to increase the representation of Black chemists.
When she asked Cláudio about specific impediments that prevent young Black people from pursuing chemistry, he said: ‘I think one of the biggest barriers that prevent people from pursuing careers in science is the lack of role models. If we only show advertisements for chemistry degrees with White people, it’s not encouraging for Black students to pursue a career there.
‘The same goes for when we visit universities; role models are needed. No one wants to be the only Black person in the department. Universities need to embrace diversity at all levels.’
George made a similar point. He emphasised the need for young chemists to surround themselves with mentors. ‘I think it is important to look for role models from the same background to help inspire you.’ When Muriel asked him which steps could be taken to increase the number of Black people pursuing chemistry as a career, he added: ‘Have more role models from different backgrounds. This sends a very powerful message to young people studying science reinforcing the message… I can do that!’
When asked about his message for Black people following in his footsteps, Jeraime said: ‘Seek out mentors, regardless of race, who can help you get there. Don’t be afraid to email them and briefly talk about your interest in the work they’ve done, what you have done, and are doing now.’
Jeraime also cited lack of representation as a barrier that prevents more young Black people from entering chemistry. ‘Lack of representation I think is the number one barrier,’ he said. ‘Impostor syndrome is bad at the best of times, but worse still if there’s no representation in the ivory tower.’
The issue of inequality in chemistry is large – far too large for a mere 752-word blog – but as we celebrate the achievements of Black chemists everywhere this week, it is clear just how much of a positive influence role models such as Cláudio, George, Jeraime, and countless others can have on the dreams and aspirations of young chemists.
>> Here are Cláudio’s, Jeraime’s, and George’s stories.
Written by Eoin Redahan and based on previous reporting by Muriel Cozier.
The Commonwealth Games has landed in Birmingham. Before the Games began, viewers were treated to an extraordinary opening ceremony (featuring a giant mechanical bull) and its artistic director, Iqbal Khan, was lauded for his ingenuity.
But such ingenuity shouldn’t surprise any of us, for Birmingham has long been a place of outsized invention. For more than 300 years, the inhabitants of this industrial powerhouse have churned out invention after invention; and its great pragmatists have turned patents into products.
Chemistry, too, owes a debt to the UK’s second city. Whether it’s the first synthesis of vitamin C, the invention of human-made plastic, adventures in mass spectrometry, or electroplated gold and silver trinkets, Birmingham has left a lasting legacy.
Here are five chemists whose innovations may have made an appearance in your life.
Alexander Parkes – man of plastic

Plaque commemorating Alexander Parkes in Birmingham, England. Image by Oosoom
Look around you. Look at the computer screen, the mouse button you click, and the wire casings everywhere. Someone started it all. That man was Alexander Parkes, inventor of the first human-made plastic.
The son of a brass lock manufacturer from Suffolk Street, Birmingham, Parkes created 66 patents in his lifetime including a process for electroplating delicate works of art. However, none were as influential as the 1856 patent for Parkesine – the world’s first thermoplastic.
Parkes’ celluloid was based on nitrocellulose that had been treated by different solvents. In 1866, he set up the Parkesine Company at Hackney Wick, London, but it floundered due to high cost and quality issues. The spoils of his genius would be enjoyed by the rest of us instead.
Sir Norman Haworth – the vitamin seer

Sir Norman Haworth
Sir Norman Haworth may have been born in Chorley, Lancashire, but his finest work arguably came after he became Director of the Department of Chemistry in the University of Birmingham in 1925. Haworth is famous for his groundbreaking carbohydrate investigations and for being the first to synthesise vitamin C.
By 1928, Haworth had confirmed the structures of maltose, cellobiose, lactose, and the glucoside ring structure of normal sugars, among other structures. Apparently, his method for determining the chain length in methylated polysaccharides also helped confirm the basic features of starch, cellulose, and glycogen molecules.
However, Haworth is most famous for determining the structure of vitamin C and for becoming the first to synthesise it in 1932. The synthesis of what he called ascorbic acid made the commercial production of vitamin C far cheaper – the benefits of which have been felt by millions of us.
For his achievements in carbohydrates and vitamin C, Haworth received the Nobel Prize for Chemistry in 1937 (shared with Paul Karrer). He was the first British organic chemist from the UK to receive this honour. Haworth even had a link to SCI, having been a pupil of William Henry Perkin Junior in the University of Manchester’s Chemistry Department.
Francis William Aston – adventures in mass spectrometry

Blue plaque for Francis William Aston. Image from Tony Hisgett
Another Nobel Prize-winning chemist from Birmingham is Francis William Aston. The Harborne native won the 1922 prize for discovering isotopes in many non-radioactive elements (using his mass spectrograph) and for enunciating the whole number rule.
For a time, academia almost lost Aston, as he spent three years working as a chemist for a brewery. Thankfully, he returned to academic life and obtained concrete evidence for the existence of two isotopes of the inert gas neon before the first World War.
After working for the Royal Aircraft Establishment during the Great War (1914-18), he resumed his studies. The invention of the mass spectrograph proved pivotal to his discoveries thereafter. Using this apparatus, he identified 212 naturally occurring isotopes.
George Elkington and John Wright – all that glitters

George Elkington patented the electroplating process developed by John Wright. Image from Spudgun67
It isn’t surprising that George Elkington should become an SCI favourite, as he blended both scientific ingenuity with business. The son of a spectacle manufacturer patented the first commercial electroplating process invented by Brummie surgeon John Wright in 1840.
Wright discovered that a solution of silver in potassium cyanide was useful for electroplating metals. Elkington and his cousin Henry purchased and patented Wright’s process before using it to improve gold and silver plating.
The Elkingtons opened an electroplating works in the city’s now famous Jewellery Quarter where they electroplated cutlery and jewellery. And they didn’t do too badly out of it. By 1880, the company employed 1,000 people in seven factories.
Alfred Bird – winging it

1906 advertisement for Birds Custard powder. Image from janwillemsen
In 1837, Alfred Bird was in a pickle. He wanted to serve his dinner party guests custard, but his wife was allergic to eggs and yeast, and egg was the main thickening agent of this delicious gloop.
Instead of serving something else, the chemist shop owner invented his own egg-free custard by substituting cornflour for eggs. His guests found it delicious and Bird’s Custard was born.
Not content with this innovation, Bird is also credited with being the father of modern baking powder. Once again, his wife’s allergies were said to be the inspiration, as he wanted to create a yeast-free bread for her. In bread and custard, true love always finds a way.
Paulina Quintanilla has developed a clever way to maximise the froth flotation technology used to extract more valuable minerals from rocks. The SCI Scholar and Poster Competition winner chatted to us about her process and how it could make mineral processing more efficient.
How would you describe your froth flotation technology in simple terms?
Froth flotation is the most widely used technology to separate valuable mineral particles from waste rock. The process is carried out in stirred tanks in which chemical reagents and air are added. Some of these reagents, called collectors, make the valuable mineral particles hydrophobic, which means that they repel water.
Consequently, the valuable mineral particles attach to the air bubbles, covering them and generating bubble-particle aggregates. The bubble-particle aggregates rise to the top of the tank, forming a froth that overflows as a mineral-rich concentrate, while the waste rock leaves from the bottom of the tank as tailings.
Froth flotation is also relevant in several other industrial applications, such as water treatment and paper de-inking.
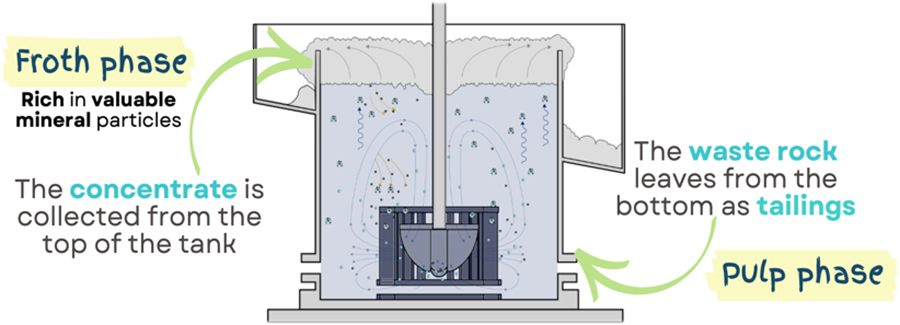
Schematic of the froth flotation process. Image by @AMPRG_Imperial.
How would you describe your froth flotation technology in simple terms?
This research focuses on optimising the froth flotation process using a control strategy called model predictive control. To this end, mathematical models were developed to represent the phenomena inside a flotation tank. These models are then used to ‘predict the future’ so that decisions can be taken now (we can control the process) to improve the froth flotation performance.
Model predictive control is a powerful optimisation strategy that has been widely used in other processes, including in the petrochemical industry, but it is still very new in the mineral processing industry.
One of the main advantages of this research is that the models are physics-based. This means that they were developed from the fundamental physics of the process rather than from data, which makes them useful under any operating conditions, for any flotation tank size. This is particularly interesting for application in the large flotation tanks used on an industrial scale.
How could this work benefit industry and make processing more efficient?
Building clean technologies for the transition to 100% green energy is creating a massive demand for a range of minerals. For example, copper mines would have to ramp up production considerably to satisfy the extra 7% predicted demand. Meeting that demand, however, is becoming more and more challenging as ores are becoming lower grade, deeper, and more complex.
This implies that there is an urgent need to optimise current processes to extract the necessary minerals and metals more sustainably and efficiently. As froth flotation is a large-scale process, even small improvements in the separation efficiency would translate into important increments in production.

Overflowing froth seen from the top of an industrial-scale tank. Image by @AMPRG_Imperial.What is the potential of this work in terms of copper recovery?
We demonstrated that improvements of between 8 to 22% in metal recovery were achieved by implementing a model predictive control strategy at the laboratory scale, revealing an untapped potential for implementation at an industrial scale. This research could serve as a promising next step for the mining industry to meet future metal and mineral demands by extracting more metal for the same amount of resources, such as water, energy, and chemicals.
>> Interested to find out more about SCI Scholarships?
Your flotation tanks are actually based in Chile. How do you operate them remotely?
I am currently implementing an online model predictive control strategy in a laboratory-scale flotation bank in Chile. I monitor and control this experimental rig from home, in the UK.
The experimental rig was automated in such a way that all the instruments (e.g. air flow meters, controllers, pumps, etc.) are connected to a module called ‘Programmable Logic Controller’. This module is then connected to a workstation computer, which I access from my laptop in the UK.
The Programmable Logic Controller allows me to obtain measurements in real-time and control the system. In this case, the measurements are used to update the mathematical models, while the system is controlled by changing the ‘revolutions per minute’ of the pumps (to change the pulp levels) and/or moving the air valves (to change the airflow rates).

Experimental campaign in 2018 – aerial view of a 300m³ froth flotation tank. Image by @AMPRG_Imperial.
Could this process be used to extract other materials? If so, which ones?
While froth flotation is widely used to separate sulphide minerals of copper, it is also used to separate other sulphides, such as those containing lead, zinc, and molybdenum.
You won an SCI Scholarship. How did you use the funds you received to develop your research?
I used the generous SCI scholarship to partially fund a two-month visit to the laboratory in Chile. I set up new connections for remote control by installing new instrumentation to make it even more automated, and I carried out preliminary online control experiments. Since then, all the control experiments have been carried out from my laptop at home.
I also used the scholarship to fund my participation in several conferences, including one in person in Athens, Greece, in 2021. I have participated in Scholar Days in 2020 and 2021, in which I presented advances in my PhD research to a wide audience. This year, I presented my PhD research results at SCI headquarters for the first time and participated in the Poster Showcase, where I won first place.

Paulina presenting at the SCI Scholars' Showcase in July 2022. Image: SCI/Andrew Lunn
What are your future plans for this innovative technology (and other potential research)?
I plan to keep up the momentum of researching froth flotation optimisation, as I believe that there is still a long way to go for improvement, particularly at an industrial scale. Model predictive control has not been widely explored within the mineral processing industry despite the fact that it has shown great potential. There is still a gap between academia and industry that should be bridged, sooner rather than later, to improve the performance of the process.
Apart from the model predictive control strategy using physics-based models (including the one I have investigated during my PhD research), many other control strategies show great potential to be tested and implemented at an industrial scale.
This is particularly applicable in mineral processing plants, as most of them collect a huge amount of data that could serve as valuable inputs for further improvement and optimisation, using novel engineering tools such as artificial intelligence and digital twins.
Paulina is part of the Advanced Mineral Processing Research Group at Imperial College London, whose research includes fluid dynamics of flotation tanks and multi-criteria decision-making for sustainable mining and mineral processing.
A range of greenhouse gas removal technologies may be necessary if we’re to reach Net Zero by 2050. In the second of our two-part geoengineering feature, Eoin Redahan looks to the sea, the sun, and mineral weathering, and at the ethical concerns such technologies raise. Missed Part One? Find it here.

‘Water, water, everywhere, nor any drop to drink.’
These famous words from Samuel Taylor Coleridge’s Rime of the Ancient Mariner aren’t the only famous part of his epic poem. The term albatross around one’s neck comes from it too.
After shooting a friendly albatross at sea, the poem’s narrator was forced by the ship’s crew to wear the dead creature around his neck – and grievous luck was to follow. Well, our blue planet has an albatross around its neck in the form of climate change.
Perhaps the solution to it lies all around us – water, water, everywhere…
An ocean of potential
In theory, we can use our oceans to pull CO2 from the air on an enormous scale. All it may take is clever intervention – potentially ruinous, albatross-shooting intervention.
Nevertheless, the World Economic Forum lays out the tantalising potential. ‘Ocean-based CO2 removal can help us achieve “net negative emissions” as the seas hold 50 times more carbon than the atmosphere,’ it says.
‘The ocean [is] a sink for nearly one third of anthropogenic carbon emissions and more than 90% of the resulting heat… If we are going to manage atmospheric CO2 levels to our advantage, we will need to leverage the ocean’s existing ability to govern the global carbon cycle.’
Frontier has targeted the development of scalable sources of alkalinity. The reasoning behind it is that with CO2 being an acidic molecule, rising CO2 concentrations could be neutralised through alkalinity. It has mentioned using mine tailings to remove up to 0.5 gigatonnes of CO2 from the air each year; but the major caveat here is that it needs to be done safely.
Planetary Technologies has ventured into this space armed, essentially, with a bicarbonate of baking soda that could draw in CO2 and sequester it for millenia.
The company explains its process: ‘We start by carefully extracting key parts of the mine tailings including recovering battery metals (like nickel and cobalt) and silica (sand) and then take the remaining purified metal salt solution into a special electrolyser. There, using clean, renewable electricity, the salt and water are split to make hydrogen (a clean, emissions-free fuel), and a pure alkaline hydroxide.
‘It’s from this point that we transport the bulk alkaline materials to our ocean outfalls site where the alkalinity is introduced to the surface ocean that then draws in CO2, sequestering it as already abundant bicarbonate and carbonate ions in seawater.’
So, by decreasing the acidity of the ocean, it would have a greater capacity to absorb CO2 from the air. The key, however, is to reduce this to a viable price point.
>> Want to read about iron fertilisation in our oceans? Rhiannon Garth Jones took a closer look here.
Mineral weathering, methane capture, and more
Mineral weathering is another contender in the CO2 removal mix. One technology that recently received $2.4m in funding is Seattle-based Lithos’ enhanced weathering process – a mineral weathering process that could capture CO2 at a gigatonne scale. According to Frontier, Lithos spreads basalt on croplands to increase dissolved organic carbon, before eventually being stored as ocean bicarbonate. The idea is to maximise CO2 removal while bolstering crop growth.
Closer to home, SAC Consulting in Edinburgh will receive £2.9m to capture the methane produced by cattle and cut emissions from the livestock farming sector; Synthetic Biology in San Francisco has received an R&D grant to synthesise a polymer within algae that is capable of sequestering atmospheric CO2 at a large scale; and Charm Industrial is converting plants into a carbon-rich liquid that is pumped underground.
To do the latter, Charm grows cellulosic biomass that captures CO2 from the atmosphere. It is then harvested, ground, and heated, before being turned into a bio-oil that is pumped underground.
Even the concrete beneath our feet could be used as a carbon sink. CarbonCure is injecting CO2 into its concrete mixes, which it says is not only comparable in cost to traditional concrete, but stronger.
And then, we have solar engineering – arguably the first technology that comes into many of our minds when we think of carbon removal. All sorts of geoengineering technologies exist in this sphere including cirrus cloud thinning, stratospheric aerosol scattering, and marine cloud brightening.
Ethical issues?
Interestingly, Harvard’s Solar Geoengineering Research Programme referred to geoengineering as ‘a set of emerging technologies that could manipulate the environment and partially offset some of the impacts of climate change’.
Therein lies the problem for many. What are the consequences of ‘manipulating the environment’, especially if these technologies fall into unscrupulous hands?
In her excellent blog for SCI on geoengineering, Rhiannon Garth-Jones referred to the Haida Corporation Salmon trial. In this trial, 120 tonnes of iron compound were deposited in the migration routes of pink and sockeye salmon in the Pacific Ocean, which resulted in a several-month-long phytoplankton bloom.
It was seen by many as a success. The phytoplankton fed fish and increased biodiversity and the iron sequestered carbon; but Environment Canada believed the corporation violated national environmental laws by depositing iron without a permit.
History teaches us that profit vs. planet tussles don’t always go the way we would like, and the consequences of these technologies going into the wrong hands could be catastrophic.
On 29 June, The World Economic Forum called for a code of conduct for ocean-based CO2 removal; and the American Geophysical Union, a group of climate and planetary scientists, is leading the way in developing an ethical framework for climate intervention engagement.
We’re all feeling the effects of climate change. As I write this piece on 19 July, it is 39°C here in Greenford, London. 39°C in London! The earth is cracking, planes are circling (because the runways are melting), and grass fires are blazing in Croydon.
On days like today, it feels like we need all the innovation we can get.
From the Black Death to the Covid-19 pandemic, great adversity has also led to great advances. So, which inventions have emerged from times of hardship? Eoin Redahan finds out.
‘World events shape innovations. The World Wars shaped innovation, and the pandemic has shaped innovation,’ said Paul Booth OBE, in his outgoing speech as SCI President.
‘It is possible to accelerate innovation – we’ve demonstrated that.’ Paul Booth OBE, outgoing SCI President at SCI’s AGM, July 2022. Image: SCI/Andrew Lunn
The pandemic taught us a lot about ourselves. It taught me that eating my body weight in sweets was a great way to destroy my teeth, and it brought home to many the futility of the five-day commute. On a more abstract level, it taught governments and policy makers just how much can be achieved in a short space of time when necessity demands it. The vaccines that swam around our veins bore testament to this.
The pandemic has shaped innovation. Nowhere is this more apparent than in medicine. It isn’t the first awful event to provide a hotbed for change, and it won’t be the last. ‘It is possible to accelerate innovation,’ Paul said. ‘We’ve demonstrated that.’
Black Death and the bird mask
As bad as the Covid-19 pandemic was, the Black Death makes it look very tame indeed. It is estimated that the Plague, which was its worst from 1346-53, took up to 200 million lives in Eurasia and North Africa.
Amid the carnage, it is also said to have given us a system to mitigate infectious diseases with which we are familiar, including isolation periods. According to Britannica, ‘public officials created a system of sanitary control to combat contagious diseases, using observation stations, isolation hospitals, and disinfection procedures.’
The terrifying doctor will see you now.
The Plague also said to have inspired greater experimentation in pharmacology. In a sense, it also helped democratise medicine, with medical textbooks shifting from Latin to the vernacular. As John Lienhard, at the University of Houston, noted: ‘Both medical and religious practice now shifted toward the laity.’
But perhaps the most memorable advance from this time was the strange, beak-like masks worn by some doctors during the Plague. These masks were a crude (and frankly terrifying) way to protect the doctors from the disease in the air. The doctors even filled these masks with herbs in an effort to protect against pathogens.
Byproducts of the war effort
Of course, wars have also led to military innovation at breakneck speed. During the American Civil War, the Minié ball was created. It spun faster than other bullets and could travel half a mile – unlike pre-Civil War bullets, which went a mere 300 feet.
Officers of a monitor-class ironclad warship, photographed during the American Civil War.
This war also led to the ironclad warship, with plates riveted together to protect against cannonballs. However, it should also be noted that many of the most interesting war-borne inventions have ended up having little or nothing to do with military application.
The ‘cotton-like-texture’ of cellucotton led to its brand name Kotex. According to this 1920 advertisement, this ‘wardrobe essential of Her Royal Daintiness’ was available at any shop that catered to women. Different times.
World War I gave us the blood bank, the Kleenex, the trench coat, and the sanitary pad. The sanitary pad has peculiar origins. In 1914, the war resulted in cotton shortages and substitutes were needed. Kimberly-Clark executives duly discovered a processed wood pulp material that was five times more absorbent than cotton, and cheaper to make. The material was used for bandages, and Red Cross nurses realised that this material could be used as makeshift sanitary pads. The company then developed a sanitary pad – branded Kotex – made from cellucotton and a fine gauze.
Synthetic rubber and Super Glue
Material substitution also led to ground-breaking innovation in World War II. According to the National WWII Museum New Orleans, in 1942 Japan cut off the US supply of natural rubber. With the demand for rubber high, US President Franklin Roosevelt invested $700m to make synthetic rubber from petrochemical byproducts at 51 new plants. By 1944, these synthetic rubber plants were producing 800,000 tonnes of the material a year.
Duct tape was developed by Johnson & Johnson during the Second World War, and by 1972 the ubiquitous tape had reached the moon. This makeshift wheel fender repair helped the Apollo 17 mission’s lunar rover to keep lunar dust at bay.
We use many other products invented during the Second World War, including duct tape, which was developed by Johnson & Johnson to keep moisture out of ammunition cases. A fellow called Harry Coover discovered cyanoacrylates – the active ingredient in Super Glue – while he tried to create a clear plastic for gun sights.
And the next time you reheat your dinner, spare a thought for Percy Spencer, the US physicist who noticed the candy bar melting in his pocket when he stood next to an active radar set. This moment of epiphany led to an invention you might know: the humble microwave.
Accelerating penicillin and vaccine rollout
Just as these trying times lead to extraordinary leaps in technology, they also lead to the large-scale rollout of said discoveries. A prime example of this is penicillin. It was first used to treat an eye infection in 1930, but it was only with the horrific fall-out of war that experiments with deep tank fermentation led to its widespread production.
A soldier recuperates in hospital thanks to penicillin in this Second World War poster. Note the not-quite-so-life-saving hospital bed cigarette. Different times, again.
The Penicillin Production through Deep-tank Fermentation paper in ACS notes that: ‘During World War II, the governments of the United States and the UK approached the largest US chemical and pharmaceutical companies to enlist them in the race to mass produce penicillin […] One of these companies, Pfizer, succeeded in producing large quantities of penicillin using deep-tank fermentation.’
The speed of development and global-scale rollout of vaccines against Covid-19 was unprecedented. Science, business and governments worked together to get the world moving again.
And, as we all know, Pfizer was back at it again during the Covid-19 pandemic. Along with AstraZeneca, Moderna, and essentially the entire pharmaceutical industry, it created vaccines that saved countless lives. Governments and policymakers were also reminded just how quickly life-saving technologies can be pushed through when needed.
But the legacy of Covid-19 treatment will stretch further, be it in nanotechnology, artificial intelligence, or other fields – who knows what else will come from it?
As Paul Booth said: ‘It is possible to accelerate innovation. We’ve demonstrated that.’
Many believe that greenhouse gas removal technologies will be necessary if we’re to reach net zero by 2050. In the first of our two-part geoengineering feature, we look at some of the difference-makers.
This week, a friend of mine played a tennis match just north of London. The game was due to take place at 18:00 but was deferred for an hour because it was 39°C. This came a day after Rishi Sunak, who may become the UK’s next Prime Minister, warned about going ‘too hard and too fast’ on net zero measures.
It’s looking increasingly likely that the implementation of environmental policies isn’t happening quickly enough; so, if we want to avoid catastrophic climate change, we will need to develop technologies that pull carbon dioxide from the atmosphere.
Mercury rising: the UK recorded record high temperatures this week.
Certainly, that’s the UK government’s perspective. ‘Greenhouse Gas Removal technology will be essential to meeting the UK’s climate change target of net zero carbon emissions by 2050,’ it said. ‘These technologies will be necessary to offset emissions from hard to decarbonise areas, such as parts of the agriculture and aviation sectors.’
Thankfully, work is underway to make this happen. And it is more than just the pang of the environmental conscience that has stirred the private sector into action. There is much money to be made from geoengineering. Indeed, a CNBC story has estimated that it could be a trillion dollar market by 2050.
The public investment has been relatively modest by some. The UK government recently pledged £54m in funding towards 15 different carbon removal technologies. But some in the private sector have dollar signs in their eyes.
A collaborative called Frontier – funded by Stripe, Alphabet, Shopify, Meta, McKinsey, and tens of thousands of businesses using Stripe Climate – has made an advance market commitment to spend an initial $925m on permanent carbon removal technologies between 2022 and 2030.
‘Models project that by 2050 we will need to permanently remove billions of tons of CO2 from the atmosphere every year,’ it states. ‘To date, fewer than 10,000 tons have been removed in total.’ The capital it has committed is designed to help companies developing carbon removal solutions to scale up.
The UK government has mentioned the need for a portfolio of carbon removal technologies to reach net zero. A cursory look reveals that there are many from which to choose, including direct air capture, the manipulation of the sea, advanced weathering, and solar engineering.
These methods are audacious, exciting, and controversial.
Direct air capture
The key, as ever, is to come up with low-carbon technologies that are both effective and economically viable. In that respect, direct air capture has emerged as a front runner. This technology often uses giant fans with filters, or chemical processes, to take CO2 from the air.
The difficulty is the amount of energy needed to power these processes and the source of this energy. The cost of removing each tonne of CO2 is also an impediment to growth – something that will need to fall for it to be implemented on a large scale.
Climeworks co-founders Jan Wurzbacher and Christoph Gebald at the Orca plant in Iceland. Image courtesy of Climeworks.
Nevertheless, significant strides have been made in recent times. Swiss company Climeworks raised US$650m in equity for its largest direct air capture plant, and last week it inked a 10-year deal with Microsoft to permanently remove 10,000 tonnes of CO2 emissions from the atmosphere on its behalf.
The company’s machines capture CO2 from ambient air by drawing air into the collector with a fan. The CO2 is captured on the surface through a selected filter material that sits inside the collectors. Once the filter is filled with CO2, the collector is closed, and the temperature is increased to 80–100°C, whereupon the CO2 is released.
And what becomes of the CO2 after that? The CO2 at its Orca facility (50km outside Reykjavík, Iceland) will be mixed with water and pumped deep underground. The carbon dioxide will then react with the basalt rock through natural mineralisation and turn into stone.
Climeworks CO2 turned into stone via Carbfix technology. Image courtesy of Climeworks.
And Climeworks isn’t the only one operating in this space. As part of the UK Government’s aforementioned £54m funding, London-based Mission Zero Technologies will receive £2.9 million to build a low-energy, heat-free way to pull CO2 from the air.
Sydney-based AspiraDAC has been backed by the Stripe Climate Fund to remove 500 tonnes of CO2 using its modular, solar-powered system. According to Frontier: ‘Its MOF (metal-organic framework) sorbent has low-temperature heat requirements and cheap material inputs, increasing the likelihood that AspiraDAC can help accelerate the production of lower-cost metal-organic frameworks which, historically, have been expensive and difficult to synthesise.’
The Stripe Climate Fund has also backed 8 Rivers Capital, LLC, and Origen Carbon Solutions, Inc to remove CO2 from the air using its direct air capture (DAC) technology. Frontier said: ‘The DAC technology accelerates the natural process of carbon mineralisation by contacting highly reactive slaked lime with ambient air to capture CO2. The resulting carbonate minerals are calcined to create a concentrated CO2 stream for geologic storage.’
Of course, direct air capture is just one of many CO2 removal solutions. In part two, next week, we’ll look at other promising technologies.
In his winning essay in SCI Scotland’s Postgraduate Researcher competition, Angus McLuskie, Postgraduate Researcher at the University of St Andrews, explains his work in replacing non-renewable and toxic feedstocks with novel sustainable catalytic processes to produce useful chemicals.
Each year, SCI’s Scotland Regional Group runs the Scotland Postgraduate Researcher Competition to celebrate the work of research students working in scientific research in Scottish universities.
This year, four students produced outstanding essays in which they describe their research projects and the need for them. In the first of this year’s winning essays, Angus McLuskie outlines his work in improving the production of urea derivatives and polyureas.
Would you risk your life for plastics and agrochemicals? You might not have to…
Urea derivatives hold a substantial global market, which is dominated by their use as fertilisers in the agrochemical sector, in addition to smaller-scale technical applications as glues, resin precursors, dyes and pharmaceutical drugs. Furthermore, polyureas are important protective coatings, with a global market exceeding £800 million a year.
Currently, urea derivatives and polyureas are produced on an industrial scale using highly toxic chemicals such as phosgene, (di)isocyanates and carbon monoxide. These reagents are detrimental to human health, as evidenced by the release of methyl isocyanate gas from the Bhopal Union Carbide factory in 1984, which led to thousands of deaths and a global outcry.
Phosgene was itself used as a battlefield chemical weapon in World War I, and is sourced from fossil-fuel-derived carbon monoxide. The result is a process with significant health and environmental impacts.
As part of a global drive to tackle climate change and move towards a circular economy, the objective of our research is to replace non-renewable and toxic feedstocks with novel sustainable catalytic processes to produce useful chemicals and materials.
>> More information about the Scottish Postgraduate Researcher competition.
In pursuit of greener methods, we have recently discovered synthetic methodologies, using a catalyst of manganese, to couple dehydrogenatively (1) methanol and (di)amines and (2) formamides and amines to make symmetrical (poly)ureas and unsymmetrical urea derivatives respectively (ACS Catal., DOI:10.1021/acscatal.2c00850).
Angus with his poster on Mn-Catalysed Dehydrogenative Synthesis of Urea Derivatives and Polyureas.
The only process byproduct, molecular hydrogen, is valuable in itself, and the non-toxic reagents of methanol or formamide can be sourced from renewable feedstocks. For example, Carbon Recycling International, an Iceland-based company, has developed methods to generate methanol industrially through the direct hydrogenation of CO2 (ATZextra Worldw., DOI:10.1007/S40111-015-0517-0). Formamides can be made from formic acid, which may be produced from biomass or CO2.
Synthesis approach
The synthesis of urea derivatives using this approach has been reported previously using iron and ruthenium catalysts, but these present individual limitations. Iron catalysts result in poor yields and substrate scope, while ruthenium catalysts are expensive and raise sustainability concerns due to ruthenium’s low abundance in Earth’s crust (Chem. Sci. J., doi.org/10.1039/C8SC00775F and Org. Lett., doi.org/10.1021/acs.orglett.5b03328).
The synthesis of polyureas via this approach has only been achieved before using a ruthenium catalyst. With a manganese-based pincer catalyst, we succeeded in making a broad variety of symmetrical and unsymmetrical urea derivatives as well as polyureas at high yields and under a low catalytic loading of 0.5-1 mol%. As the third most abundant transition metal in Earth’s crust, manganese is much cheaper than ruthenium, which improves the economic viability of the process for industrial applications.
Breaking new ground?
This is the first example of the synthesis of polyureas from diamines and methanol using a catalyst of an Earth-abundant metal. We have demonstrated for the first time the synthesis of a potentially 100% renewable polyurea from methanol and a renewable diamine Priamine, which is commercialised by Croda. This could be of interest to emerging businesses for making bio/renewable plastics.
Angus hopes his research will help us develop urea-functionalised agrochemicals and pharmaceutical drugs in a more efficient, greener way.
This initial proof of concept is exciting, but there are challenges to overcome for commercialisation. Evidently, the cost is important, and since the catalyst is much more expensive than reactants, such as amines and methanol, the cost is directly linked to the catalyst’s activity; a homogeneous catalyst that is non-recyclable and offers a turnover number of 100-200 makes the process expensive.
We are now focusing our efforts on enhancing the efficiency of the catalyst to increase cost-effectiveness, which will also allow us to make commercially important urea-functionalised pharmaceutical drugs and agrochemicals with greater efficiency and reduced impact on the environment, human health, and economy.
What is the verdict on the 100% sustainable fuel Formula 1 plans to use in its cars, and is the new E10 fuel this season doing any good? We asked David Bott, SCI’s Head of Innovation.
Beware of Greeks bearing gifts. This phrase comes from Virgil’s Aeneid, and it refers to the Greeks’ gift of a giant wooden horse to their enemies during the Trojan War. But this was no gift at all.
This warrior-filled, hollow wooden horse that the Trojans wheeled inside the gates of Troy was a ploy from the Greeks to get inside the city’s impenetrable city walls and ambush their enemy. It turned out things weren’t quite what they seemed.
Just as Trojans became wary of giant wooden horses, we should be wary of Net-Zero pledges. These promises seem impressive but, if you look inside, they might not be quite as beneficial to the environment as advertised – at worst, they could be hollow.
Whenever an organisation talks of carbon credits, makes a vague reference to biomass or a grand pledge with little detail, it is worth closer investigation.
Formula 1 recently made a sustainability pledge of its own. Following its decision to use E10 fuel in the cars this season (a mixture of 90% fossil fuel and 10% ethanol), it has announced plans to use a 100% sustainable drop-in fuel in its vehicles as part of its plans to reach Net-Zero by 2030.
On first reading, the terms Net-Zero and Formula 1 don’t sit easily together. Isn’t this the sport where 20 cars can burn more than 100kg of fuel each per race? The same travelling circus in which cars, teams, and drivers are flown and ferried all over the world for more than eight months of racing?
By its own calculation, in a November 2019 report, Formula 1 is responsible for 256,551 tonnes of carbon dioxide emissions each year. To put that figure into perspective, you would need to drive for 6,000km in a diesel car to generate a single tonne of carbon emissions – multiply that by 256,000, and Net-Zero feels some distance away.
What about the E10 and proposed drop-in fuels?
Both Formula 1’s new fuel and pledges merit closer inspection. Regarding the move to the E10 fuel in Formula 1 cars, David Bott, SCI’s Head of Innovation, wasn’t exactly gushing.
‘E10 is an evolutionary backwater – adding just 10% ethanol does nothing for emissions,’ he said. ‘A quick enthalpy calculation shows the energy in the fuel has decreased, so you need more.’
The proposed move to a ‘100% sustainable drop-in fuel’ used in standard internal combustion engines is seen by many as a positive move. Formula 1 says the fuel will be made using components from either carbon capture, municipal waste, or non-food biomass.
Each of these ‘components’ on its own is worth exploration. For example, what types of municipal waste do they mean, which types of non-food biomass are they talking about, and what about the manufacturing process?
Biomass fuel is controversial due to concerns over carbon sequestration and land use.
The passage of time will reveal more but, again, David has questioned the green credentials of the proposed fuel. He said: ‘What Formula 1 is proposing to do is analogous to sustainable aviation fuel – to make octane from a non-fossil source of carbon.’
‘[To do this], you can use biomass or “synthetic”, which basically means distillate plastic waste. It is effectively using fossil carbon that was used for something else; so, it doesn't make the situation any worse, but neither does it really contribute to lowering emissions. It’s just short-cycle carbon.’
Freight with difficulty
The mention of aviation is pertinent when it comes to Formula 1. The emissions generated by the 10 teams’ vehicles across 21 Grands Prix, including races and testing, account for just 0.7% of Formula 1’s total emissions. But by far the biggest contributor to its CO2 emissions are logistics – the movement of equipment from venue to venue by land, sea, and air.
The equipment used in Formula One must be transported from continent to continent by sea, land, or air.
After that comes business travel at 27.7%, which includes the air and ground transportation of all individuals, as well as the hotel footprint from all Formula 1 teams’ employees and major event staff. So, it’s clear that the main environmental problem isn’t the fuel used during the races; it is all of the other transport emissions.
To be fair to Formula 1, the sport has made an effort to make operations greener, including powering its offices using 100% renewable energy and taking measures to make freight more efficient.
However, any claims that it is motoring to Net-Zero by 2030 need to be chased with a liberal swig of scepticism. A Net-Zero 2030 goal provides a nice headline, but how you get there is the story.
Is it dipping your finger into a glistening bowl of mercury? Is it symmetry? Is it the patterns of crystal growth or is it to be found in nature – in the neatness of evolution? In his thought-provoking SCItalk, Philip Ball explored the beauty of chemistry.
When you write fiction, you’re supposed to wake all the senses. So, don’t just tell readers what something looks like. Tell them how it feels. Tell them how it sounds. Tell them how it tastes. For beauty exists in the smell of perfume as someone walks by, just as it resides in the colours of bloom. One of the beauties of chemistry – like nice writing – is that it also evokes all of the senses.
That was what drew Philip Ball to chemistry: the profusions of colour, the explosions, the reek of sulphur, dipping his finger into a bowl of mercury as a lad and wondering how this dense, silvery liquid hadn’t made his hand wet.
And yet, chemists – and scientists in general – seem to have a complicated relationship with beauty. Part of this is down to what different groups see as beautiful. ‘When scientists talk about beauty,’ he said, ‘they think they’re talking about what artists are, but they really aren’t.’
A chemical garden formed from copper nitrate in sodium silicate solution by Yan Liang and Wenting Zhu.
For a physicist, an equation might capture the essence of beauty. For a chemist, it might be the shape of a crystal growth formation. Ball argued that chemists tend to be Platonists – that they locate beauty in symmetry (for Plato, he added, art was too messy ever to be beautiful).
Chemistry’s reputation as a staid science isn’t helped by the fact that it has long hidden its light from the world. Much beauty is confined to those who view it under microscopes. It is only relatively recently – with the proliferation of high-resolution imagery – that the public has finally looked upon the beauty of chemical gardens, processes, and configurations in all their stunning detail.
Even so, despite the bewitching quality of seeing copper hydroxide billowing like a jellyfish, and the jagged architecture of lead formations, much of chemistry’s beauty lies in its dynamism, rather than the confines of the still frame.
The inspiration of nature
And yet, it wasn’t ever thus. Chemistry in bygone centuries was viewed slightly differently. ‘Of the chemistry of his day and generation, [the German philosopher] Kant declared it was a science, but not Science,’ Ball noted.
Similarly, in Frankenstein, Mary Shelley painted chemistry in a different light to how it is seen today. ‘Chemistry is that branch of natural philosophy in which the greatest improvements have been and may be made,’ her character, Professor Waldman, said.
The sheer beauty in science has long been appreciated, as is seen in this cyanotype photogram made by Anna Atkins in her 1843 book, Photographs of British Algae: Cyanotype Impressions.
So, why the small s? Why was it seen, not as a soft science, but one with a softer underbelly – like a stone-faced steel worker who secretly writes poetry? Perhaps it has to do with the link to creation. ‘Chemists display, arguably, the greatest creativity in the sciences,’ Ball said. ‘[They have] the urge to make stuff.’
This creativity is often guided by the beauty of the natural world. Ball argues that some scientists are guided by the sheer beauty of nature, by finding the unexpected in things we have seen so many times before.
On the screen, he put up a picture of what looked like the intricate component of a motor, which turned out to be the natural motor structure within bacteria driving its very survival. He mentioned the pigments within flower petals, so delicately tuned by evolution.
An extraordinary bacteria motor (left). Image from paper on: Structural basis of assembly and torque transmission of the bacterial flagellar motor. Created by Zhejiang University researchers..
Simply put, the elegant solutions found by nature are inspiring. ‘It made me think about what Einstein said,’ he added. ‘The Theory of Relativity was so beautiful to him that he believed nature had to work this way.’
And some chemists are drawn by a different type of aesthetic: the beauty of the method. Just as a football fan might rhapsodise about the arc of a perfectly struck free-kick as it curves beyond the keeper’s reach, some chemists see something in the process. ‘For some chemists, there’s a beauty in the synthesis,’ Ball said; and other chemists, he added, will have their own aesthetic responses to an approach, be it elegant or otherwise.
Why shouldn’t the work of a chemist be driven, in part, by beauty? And why should the arbiters of the aesthetically pleasing be confided to the arts? For Philip Ball, the chemical world is one of artistry, dynamism, and beauty. For him, science provides a new lens, new tools for seeing, and new ways for looking at the world around us.
‘Science doesn’t de-enchant the world,’ he said. ‘On the contrary, it re-enchants it.’
Philip’s book, The Beauty of Chemistry, is published by MIT Press.
What makes chilli peppers so spicy and how do they help with pain relief? The SCI Horticulture Group explained all ahead of their appearance at BBC Gardeners’ World Live in Birmingham from 16-19 June.
This June, the SCI Horticulture Group will tell the public all about the hidden chemistry behind their favourite fruit and vegetable plants. One of the main plants they will feature at the National Exhibition Centre is the humble chilli pepper – and these famous fruit-berries conceal more secrets than you might think…
Where does the chilli originate?
The chilli pepper (Capsicum spp.) is a member of the Solanaceae, the plant family that includes edibles such as potatoes, tomatoes, aubergines, but also poisonous plants such as tobacco, mandrake, and deadly nightshade.
Who brought the chilli pepper to these shores?
The chilli was brought to Europe in the 15th century by Christopher Columbus and his crew. They became acquainted with it on their travels in South and Central America and, shortly thereafter, to India via the Portuguese spice trade.
How varied is the genus?
Of the 42 species in the capsicum genus, five have been domesticated for culinary use. Capsicum annuum includes many common varieties such as bell (sweet) peppers, cayenne and jalapenos. Capsicum frutescens includes tabasco. Capsicum chinense includes the hottest peppers such as Scotch bonnet. Capsicum pubescens includes the South American rocoto peppers, and capsicum baccatum includes the South American aji peppers.
From the five domesticated species, humans have bred more than 3,000 different cultivars with much variation in colour and taste. The chilli and bell peppers that we eat are the fruit – technically berries – that result from self-pollination of the flowers.
>> The SCI Horticulture Group brings together those working on the wonderful world of plants.
And we eat a lot of them?
Today, chilli peppers are a global commodity. In 2019, 38 million tonnes of green chilli peppers were produced worldwide, with China producing half of the total. Spain is the largest commercial grower of chillies in Europe.
Capsaicin helps give chilli peppers their heat
What makes chillies hot?
Capsaicin is the main substance in chilli peppers that provides the spicy heat. It binds to receptors that detect and regulate heat (as well as being involved in the transmission and modulation of pain), hence the burning sensation that it causes in the mouth.
In humans, these receptors are present in the gut as well as the mouth (in fact, throughout the peripheral and central nervous systems) – hence the after-effects of eating too much chilli. Capsaicin, however, is not equally distributed in all parts of pepper fruit. Its concentration is higher in the area surrounding the seeds.
>> Get tickets for Gardeners’ World Live 2022 and pop by our stand to say hello!
How do you measure chilli heat?
The Scoville Heat Unit Scale is used to classify the strength of chilli peppers. Scoville heat units (SHU) were named after American pharmacist Wilbur Scoville who devised a method for rating chilli heat in 1912.
The ludicrously hot Dragon’s Breath chilli
This method relied on a panel of tasters who diluted chilli extract with increasing amounts of sugar syrup until the heat became undetectable. The greater the dilution to render the sample’s heat undetectable, the higher the SHU rating. Pure capsaicin measures 16,000,000 SHU.
The capsaicin content of chilli peppers varies wildly, as is reflected in the SHUs of the peppers below:
- Bell (Sweet peppers) = 0 SHU
- Jalapeno = 5,000 SHU
- Scotch Bonnet = 100,000 SHU
- Naga Jolokia = 1,040,000 SHU
- Carolina Reaper = 1,641,183 SHU
- Dragon’s Breath = 2,480,000 SHU
Dispersal vs. protection – why do chillies contain capsaicin?
The seeds of chillies are dispersed in the wild by birds who do not have the same receptors as mammals and, therefore, are unaffected by capsaicin. Perhaps chillies have evolved to prevent mammals from dispersing their seeds?
Capsaicin has also been shown to protect the plant against fungal attack, thus helping the fruit to reach maturity and the seeds to be dispersed before succumbing to rot. This antifungal property can also be put to good use in helping to preserve foods for human consumption.
How did chilli help win a Nobel Prize?
Capsaicin was pivotal in the research that led to the award of the 2021 Nobel Prize in physiology and medicine to David Julius and Ardem Patapoutian for their discoveries of receptors for temperature and touch.
The two US-based scientists received the accolade for describing the mechanics of how humans perceive hot, cold, touch, and pressure through nerve impulses. The research explained at a molecular level how these stimuli are converted into nerve signals, but the starting point for the study was work with capsaicin from the humble chilli pepper.
Capsaicin in medicine
Capsaicin is used as an analgesic (a pain reliever) in topical ointments, nasal sprays, and patches to relieve chronic and neuropathic pain. Clinical trials continue to investigate the potential of capsaicin for a wide range of additional pain indications and as both an anti-cancer and anti-infective agent.
>> Special thanks to Neal Price from Chillibobs, Martin Peacock of ZimmerPeacock, Hydroveg, and The University of Reading Soft Fruit Technology Group for supporting the work of the SCI Horticulture Committee at BBC Gardeners’ World Live.
>> Our resident gardening expert, Geoff Dixon, provides plenty of gardening tips on the SCIBlog.
Have you ever seen a snowflake up close? Have you smelt fertiliser on a country drive? Chemistry is the most sensuous of the sciences, and it may just be the most beautiful too. In our latest SCITalk, Dr Philip Ball showcases the breathtaking beauty of chemistry.
Main image: A chemical garden formed from copper nitrate in sodium silicate solution by Yan Liang and Wenting Zhu.
Even the most disciplined of us falls into these rogue states from time to time, minutes of total absorption unrelated to work or duty. For some, it is the humble cat video. For others, it is the endless tapestry of Twitter.
Crystals of nicotinic acid by Yan Liang and Wenting Zhu.
For me, this morning, it was a time-lapse video of crystal growth patterns. The world temporarily stopped moving as I fell headlong into high-resolution pictures of icy fronds appearing and clusters of spikes combining to form crystalline towers. Who knew potassium nitrate, ammonium chloride, and monopotassium phosphate could be so beautiful?
It turns out, Dr Philip Ball did. He knows all about the beauty of chemistry – from its profusions of colour to the hypnotic beauty of snowflakes forming.
Oxygen bubble from decomposing hydrogen peroxide by Yan Liang and Wenting Zhu.
Dr Ball argues that chemistry is the most sensuous of the sciences. Which of us hasn’t smelt the stink of sulphur or the sting of ammonia in our nostrils? When he unveils vivid, other-worldly pictures of chemical gardens, or even when we see a close-up of water being added to a bowl of M&Ms, it’s hard to disagree with his view.
This Wednesday evening, 25 May 2022, Dr Ball will deliver his SCI Talk about the beauty of chemistry and his book of the same name, which he put together with photographers Yan Liang and Wenting Zhu. Using microphotography, time-lapse photography, and infrared thermal imaging, they have captured astonishing photos of chemical processes.
They have captured a beauty seldom seen, except by chemistry’s day-to-day practitioners. They show us the chemistry of champagne in a new light and the transformations of evaporation and distillation. They unveil the strange world of chemical gardens – from the blue tendrils of copper nitrate in sodium silicate solution, to the silky precipitation of silver chromate.
Precipitation of silver chromate by Yan Liang and Wenting Zhu.
Some defend the beauty of science by conflating it with the pursuit of truth. As the famous snippet from Keats’ Ode on a Grecian Urn goes: ‘Beauty is truth, truth beauty.’ Yet, it’s clear that the beauty of chemistry does not need to be defended in such abstract terms. It’s there in champagne bubbles and the deft configurations of a snowflake. You just need to look into a microscope - or plunge mind-first down a YouTube rabbit hole.
Register here to watch the Beauty of Chemistry SCItalk this Wednesday 25 May 2022.
An Artificial Intelligence tool that could change the way we treat heart disease wowed the judges at this year’s Bright SCIdea competition. Now that the dust has settled, we asked Raphael Peralta, from the winning CardiaTec team, about winning the competition, the need for this technology, and tips for future participants. After winning this prestigious competition and coming away with the £5,000 first prize, the future is bright for co-founders Raphael Peralta, Thelma Zablocki and Namshik Han. So, how do they reflect on the story so far?
Team CardiaTec (UK)
Tell us about CardiaTec
Cardiovascular disease is the world’s leading cause of death, and affects countless lives. Despite this, investment and innovation within the space has been severely stagnated, especially in comparison to fields such as oncology. The current treatment landscape remains unchanged, and treatments are most often prescribed in a standardised, one-size-fits-all approach. However, people are fundamentally different, and as shown by the Covid-19 pandemic, similar groups of people can experience a disease in a significantly different manner, and as such it is very important to understand biological processes at a patient level to produce effective therapeutic outcomes.
CardiaTec is leveraging artificial intelligence to structure and analyze large scale biological data that spans the full multiomic domain. This allows for a comprehensive understanding of disease pathophysiology to better develop novel and effective therapeutics for cardiovascular disease.
Casting your mind back to the moment you were announced the winner of Bright SCIdea 2020, what were your initial thoughts?
We thought we had a good opportunity to win it, but obviously when it was announced, it was a great feeling. Winning this competition is a further validation that what we are generating has real world value.
It was a great judging panel, with a breadth of experience across drug discovery and the pharmaceutical industry. We were up against immense global competition and the fact that we won shows that there’s a need for novel innovation in the cardiovascular space to ultimately drive the development of new therapeutics that are going to help change people's lives.
How did you think of the idea? Was there a ‘eureka’ moment?
The way the initial idea came about was through the identification that the cardiovascular space had a massive unmet need compared to other spaces such as oncology. I had worked with a cardiovascular company doing some consulting work and this is where it came to light.
In combination, multiomic techniques are becoming increasingly accessible in line with technological developments, which have made processes of next generation sequencing and proteomic profiling increasingly cheaper. These processes generate large amounts of data, which then lend themselves to applications of machine learning to derive biologically meaningful insights. These process, although becoming increasingly familiar in areas such as oncology, are highly underrepresented in cardiovascular disease, and thus there spans opportunity to develop completely unique and novel insights.
How does the technology work?
Here, CardiaTec uses data across genomics, epigenomics, transcriptomics, proteomics, and metabolomics, to generate novel biological insights with the help of AI and machine learning applications. Taking these many ‘omics’ into consideration is what defines a ‘multiomic’ approach. Biology is complex, and trends require full multiomic assessment to truly understand where dysregulation of specific processes is occurring, to then inform the best means of intervention.
CardiaTec is developing a platform, which with time will grow to become one of the most comprehensive foundations of cardiovascular disease biology. Results and outcomes are iteratively incorporated into the model, and new hypotheses are tried and tested across a range of pre-clinical settings. Collectively, CardiaTec aims to generate novel drug targets that can be used to help reduce the burden of disease in current and future patient population.
In the process of getting to the final, there were several opportunities to engage with entrepreneurs, investors, business leaders, and experts in intellectual property (IP). Can you share key takeaways from these sessions?
One of the most important things you can do is speak to people. Every business starts from an idea. As you start developing, you change and refine the business model. We take every chance to engage with people who have industry experience. It’s really important that we take the advice of these people on board; this is especially true in the field of biotechnology where you take risks across the technology side, the commercial side, and the biological side. It takes a lot of experience to mitigate those risks.
How difficult has it been taking that idea and turning it into a viable business proposition?
Thelma and I came out of the MPhil in Bioscience Enterprise at the University of Cambridge. It gave us this really strong foundation to start building. We also had the biological knowledge from our previous degrees. This framework, where we had key opinion leaders and great people in the field with whom we could bounce ideas off, was the first step. We saw that the idea was really positive and was received well by a lot of people. So, we thought: ‘we’re onto something’.
When building a biotech company, if you’re not passionate about it and don’t want to spend a lot of your time dedicated to the project, then it’s not going to take off. You need to be there to make changes, and really embrace and understand where you believe it’s going to go in line with the advice you've been given and the insights that you have generated.
We’re not only interested in understanding the intricate nature of biology. We’re also interested in how this has real life application in changing people’s lives. Every person we speak to has been affected in some way by cardiovascular disease.
I noticed that your presentation was really polished. Do you have any tips for people presenting in the final?
We’ve presented a lot of times so I think practice makes perfect. With a presentation, you need to be able to tell a story. It’s all about the storyline and building that image. You have to take care and be diligent in the process. Take time to make sure everything is structured correctly and that the story flows. Don’t be afraid to present to a lot of people who will give you advice. Take the time to make the amendments and run it through again and again, and see what the response is. So, take your time on the presentation to get your story across.
You were both very calm when the judges’ questions came. How did you prepare for these questions?
Out of this Cambridge network, the people we spoke to all asked the right questions. You see the pattern of these questions. They all want to know similar things. So, once we identified that pattern, we wrote down the questions that were important from our conversations and we practiced responses to these questions, which were by this point, fully embedded into the company’s business model; which then lends itself to an insightful, actionable response.
How are you going to use the £5,000 prize money and what’s next?
We’ll put the prize money towards refining of some of our technology. In terms of what’s next, Thelma (Zablocki), Namshik (Han), and I are dedicated to this company. We want to see it through and eventually make a drug that ends up reaching patients. This will take a long time.
To see that in the real world, where someone’s getting prescribed a drug that you discovered would be incredible.
>> For more on this year’s Bright SCIdea final, go to: https://www.soci.org/news/2022/3/bright-scidea-final-2022.
Re-using waste materials and converting them into chemicals will help us create a closed-loop system. Ahead of the SCI Engineering Biology symposium on 23 May, Martin Hayes, Biotechnology Lead at Johnson Matthey, spoke about some exciting approaches and the challenges involved in making the low-carbon transition.
The journey to Net Zero is well underway, with a number of countries already committed to Net Zero by 2050. To achieve this ambitious goal, companies and governments must take a new approach to waste, shifting from linear processing to a circular model.
This involves recycling and reusing products to create a closed-loop system that uses fewer resources and reduces waste, pollution and carbon emissions. As we journey towards Net Zero, these ‘circularity’ principles are increasingly embedded in the research and design of products.
Re-using waste from chemical processes
As a leader in sustainable technologies, Johnson Matthey (JM) is striving to help the chemical industry transition. Martin Hayes, Biotechnology Lead, explains: ‘More and more companies are starting to move away from linear chemical processes to circular ones, which is definitely a step in the right direction.
‘They’re looking at how the waste from chemical processes may be the source for biological processes. Biological entities such as enzymes or organisms can even recover precious metals from waste streams, maximising value while reducing waste.’
>> How are young chemists tackling climate change? Read more in our COP26 review.
In other cases, gas fermentation can upgrade waste products, particularly carbon dioxide and hydrogen, and convert them into chemicals. Hayes explains: ‘In this instance JM joins biology and chemistry to get the desired end product without affecting the customer experience, but making the process much cleaner.’
Fermented food waste could be converted into chemical building blocks.
Food waste is another contributor to greenhouse gas emissions. A circular approach may consider fermenting food waste to convert it into useful chemical building blocks. ‘What is valuable about this is that these chemicals are not produced from virgin fossil material,’ he adds.
Collaboration and feedstock issues
To realise the potential in these technologies and new businesses, it’s important to take a collaborative approach and for multi-disciplinary teams to work together. Hayes continues: ‘We know that getting the biology to the end product requires engineers, chemists, microbiologists, and biochemists – different scientists working together with commercial expertise to make a product that is sustainable, has a low environmental footprint, and is still profitable.
‘We work collaboratively in partnership because we recognise we need to develop these solutions in ways that reflect the needs of each client and the broader society.’
But the scale of the issue shouldn’t be underestimated. On the one hand, those biological entities will require engineering to become efficient catalysts, working selectively with less-than-ideal feedstocks under demanding reaction conditions. On the other hand, scaling up and optimising processes such as fermentation can be resource intensive and involve large volumes.#
Johnson Matthey will be Platinum sponsors for the upcoming Engineering Biology symposium | Editorial image credit: Casimiro PT / Shutterstock
This type of catalyst customisation and process intensification calls for a multi-disciplinary team: bioinformaticians, molecular biologists, chemists and chemical engineers working together.
While the UK leads in renewable technologies, it is also important to think in terms of connected systems rather than isolated applications of technology. That broader perspective in a circular system will get us towards Net Zero and is embodied by the SCI’s symposium on Engineering Biology with which JM is proud to be associated as a (fittingly) Platinum sponsor. This is a topic which is entirely consistent with, and supportive of, JM’s vision of a cleaner, healthier world.
>> Sign up here for SCI's Engineering Biology – applications for chemistry-using business on 23 May.
>> How do we move to non-fossil fuel feedstocks? Here’s our report on the Parliamentary & Scientific Committee Discussion Meeting on 28 March.
Interested in a career in chemistry publishing? Then see how Bryden Le Bailly, Senior Editor at Nature, navigated the path from academia to science communication.
Tell us about your career path to date.
I am a Senior Editor at Nature magazine, overseeing what we publish at the chemistry/biology interface. I completed a MSci in Chemistry at the University of Bristol, followed by a PhD in Organic Chemistry at the University of Manchester in which I looked at signalling with synthetic systems in membranes. I was always interested in education generally, and a great teacher of mine told me Chemistry would have enough to keep me engaged. She wasn’t wrong.
Bryden Le Bailly, Senior Editor at Nature magazine
A short post-doctoral position let me carry on research for a year, but I became more certain that a career in academia wasn’t for me. I enjoyed the idea of research more than its realities, and academia didn’t really work with other life choices I wanted to make. Editorial work suits this balance far better while staying close to the science.
Coupled with my interest in science communication, it looked like a good fit. To read and discuss exciting, cutting-edge research didn’t seem too bad a way to make a living. I looked into editorial jobs and, after discussions with a former editor in the Bristol Chemistry department, I started applying for positions at Nature journals. A locum position at Nature Nanotechnology led to me applying for the permanent position at Nature, where I’ve been for a little over five years.
What is a typical day like in your job?
The core of the job is deciding which submissions to review and publish. So, I read, a lot. The areas I cover comprise how molecules are made and how they can be used to interrogate biology or as therapeutic leads, as well as biochemistry, membrane protein biology, and a few other bits and pieces.
If that sounds like a wide range of topics, it is! It’s the same for all Nature editors. This keeps the job varied and interesting. The rest of the job stems from the papers I handle: overseeing peer review, taking decisions post-review, and what reviewer requests need addressing before we can proceed.
This all involves discussions with my fellow editors. In addition, I speak to Principal Investigators (PIs) and other lab members about work coming out of their labs that might be suitable for Nature.
After we decide we’ll publish something, I look for other ways we can promote the work. I pitch papers we are publishing for associated coverage in News & Views, features, or to go on the magazine cover.
Finally, Nature editors commission reviews and perspectives on topics we think are important and timely, and we discuss with our magazine editors news or topics that we believe should be covered journalistically.
Which aspects of your job do you enjoy the most?
Travelling for the job has to be one of its best perks. I manage to take around five to six trips a year, locally and internationally, to conferences and labs. Discussing brand new science one-on-one with the foremost experts in that field is a massive privilege.
However, I also enjoy supporting early-career researchers to publish in Nature and guiding them through our selection process and expectations. A longer-term way I have looked to support early career researchers (ECRs) is by delivering writing and publishing Masterclasses.
What is the most challenging part of your job?
Saying no to about 90% of what gets sent to my desk at Nature, despite it being (mostly) great science.
>> Excited about a career in next generation drug development? Read how Rachel Ellis became involved in Rachel's Careers for Chemistry blog.
How do you use the skills you obtained during your PhD/Postdoc in your job?
A good knowledge of organic chemistry and chemical biology is very helpful, not only for assessing manuscripts but also to advise on standards for Nature and the rest of the Nature portfolio. I am glad I chose research projects that required me to learn a range of techniques and delve into lots of different areas. Some of the more tangentially related areas to my studies are core responsibilities for me in my job now.
Which other skills are required in the work you do?
An interest in a breadth of science and willingness to learn are key. You will be exposed to areas you had previously never appreciated or knew existed in this job, and it is important to understand every submission from all its angles, and quickly.
This involves effective communication with other editors. Communication and learning skills also come into play when you’re out and about, where you might discuss 15 different subjects over a poster session at the end of a long day, or during a visit to an institute. Finally, editors need a good eye for detail.
Bryden has used his background in organic chemistry to forge a career in publishing.
Is there any advice you would give to others interested in pursuing a similar career path?
Firstly, the pace of the job and its expectations are very different from research. Looking at a manuscript from a scientific and editorial standpoint are two very different things. Consider if you have a critical eye when reviewing papers for a journal or reading the literature.
If you can explain to your colleagues or friends why a piece of research is exciting or ground-breaking, this is a good starting point. However, my principal advice would be to talk to editors.
We go to conferences and are happy to discuss the job in more detail. When I first applied for editorial roles, it was helpful to discuss the position with a former editor. When I didn’t get the jobs I applied for, one of the interviewers called me to explain and encourage me in the right direction. This experience was invaluable in getting me to where I am today.
>> Suze Kundu went from academia to presenting TV shows on the Discovery Channel. Trace her storied career path in Suze's Women in Chem blog.
In the first of our new Careers for Chemistry Postdocs series, Rachel Ellis, Senior Client Proposal Coordinator at drug development company Quotient Sciences, speaks about putting her chemistry skills to the test in a new setting and integrating scientific knowledge with people skills.
Rachel Ellis, Senior Client Proposal Coordinator at Quotient Sciences
Tell us about your career path to date
In my current role as a Senior Client Proposal Coordinator, my primary responsibility is to support the Business Development team by collating technical information from the different business units at Quotient Sciences to prepare proposals that meet the prospective clients’ needs, spanning multiple disciplines of drug development.
I work with subject matter experts in Active Pharmaceutical Ingredient (API) synthesis and scale-up, carbon-14 isotope labelling, formulation development, analytical services and drug product manufacturing to generate complex written proposals for clients looking to accelerate their drug development programmes.
I started my career in chemistry with a Master’s degree from The University of York, which encompassed a year-long industrial placement with a speciality chemicals company in the Netherlands. This was a fantastic opportunity to put my chemistry skills to the test for the first time in an industrial setting and informed my decision to explore a career in chemistry outside of academia.
Following completion of my degree, I started working life as a Research Chemist within a global contract research organisation (CRO). The position was a perfect fit for my interests at the time; it was organic synthesis-focused, within the pharmaceutical sector and involved face-to-face interaction with clients.
After 18 months in the role, I identified my strengths in communication and relationship building so took the decision to pursue a career outside of the laboratory, moving into scientific recruitment where I could apply my scientific knowledge and soft skills in equal measure. I spent four years in scientific recruitment where I developed an array of new skills including networking, negotiating, influencing, account management, people management and performance evaluation.
Following a busy four years, I decided to take some personal time to focus on priorities outside of my career and embarked on a twelve-month career break. This was a fantastic opportunity to reassess my skills, interests and objectives, which ultimately brought me into my current role in proposal development. The position perfectly integrates my scientific knowledge and people skills and offers opportunities for continuous development in a dynamic sector.
What is a typical day like in your job?
A typical day as a Proposal Coordinator involves the evaluation of proposal requests from clients, technical discussions with subject matter experts to define project requirements, the preparation of comprehensive proposals including technical writing, pricing assessments and resource planning and any additional client engagement activities to support the proposal award.
Typically, I would lead the preparation of several proposals at any one given time which may include one or more drug development services.
Rachel Ellis seeks to help deliver life-changing medicines in her current role.
Which aspects of your job do you enjoy the most?
I particularly enjoy engaging with new clients to discuss how we can support them to accelerate the delivery of life-changing medicines to the market with greater speed and efficiency. I also enjoy the diversity of tasks involved in my role (scientific discussions, technical writing, pricing activities and project planning) and the balance between working independently and collaboratively as a team.
What is the most challenging part of your job?
As my role involves supporting multiple proposals at any one given time, time management and prioritisation can be challenging to ensure both internal and external deadlines are met. Organisational skills and open communication are key to ensuring projects are delivered on time and client engagement is maintained.
>> Interested in joining SCI’s Young Chemists’ Panel? Find out more on the Young Chemists Panel's webpage.
How do you use the skills you obtained during your degree in your job?
The breadth of scientific knowledge gained from my degree has provided a robust foundation for my current role and enables my participation in technical discussions across multiple scientific disciplines. Report writing, time management and attention to detail are also key skills that I now apply on a day-to-day basis.
Which other skills are required in the work you do?
My current role requires collaboration between many individuals (both internally and externally) across a multitude of disciplines, including technical experts, project managers, business development teams and financial teams.
Strong interpersonal skills are key to ensuring all parties are engaged and aligned in decision making processes. Effective communication skills are also the foundation for a career within any client-facing environment.
Is there any advice you would give to others interested in pursuing a similar career path?
In general, I would strongly advise investing time to evaluate the variety of roles available within the science sector. Don’t be afraid to explore opportunities outside of the norm. Over the course of my career to date, my eyes have been opened to the breadth of roles available within science that are not necessarily laboratory-based, such as regulatory affairs, quality assurance, medical communications and commercial positions.
I would also advise regular self-evaluation to assess your strengths and areas of interest at any given time to assist in the building of a personalised career development plan. This will help to focus your attention on opportunities to develop the skills you need and seek out exposure to relevant activities either within your current organisation (i.e. attending client calls/visits or developing interpersonal skills through participation in cross-departmental activities) or through voluntary work and networking.
>> Interested in a career in science communication? Read Suze Kundu’s inspiring story.
We caught a tantalising glimpse of the next generation wearable technology at this year’s Bright SCIdea challenge final.
When we look at our FitBits or Apple Watches, we wonder what they could possibly monitor next. We know the fluctuations of our heartbeat, how a few glasses of wine affect our quality of sleep, and the calories burnt during that run in the park. But what’s next?
If the amazing wearable devices pitched by just three of our Bright SCIdea finalists are anything to go by, then we can look forward to not just next generation health monitoring but possible in-situ treatment too.
Measuring stress and managing diabetes
In recent times, medics have learnt far more about stress and its effect on our health. Indeed, stress was the focus of Happy BioPatch (from Oxford University and Manchester University) technology. The second place team has incorporated an IP-protected enzyme within a patch that measures your stress levels (by detecting the levels of cortisol in your sweat) throughout the day.
This information migrates from body to phone and notifies you if your stress levels are too high. One of many exciting aspects of this technology is that it could be used by physicians to check if patients need treatment for depression and prevent the serious consequences of stress. As one of the judges said, ‘I like it because it’s preventative.’
From mental health to physical health, two of the other finalists use wearable devices to address maladies in in-situ. BioTech Inov, from the University of Coimbra in Portugal, has developed plans for a subcutaneous biomedical device that tracks the blood sugar levels in diabetes patients. This technology would enable the wearer to track their blood sugar levels and let them know if trouble is lurking.
The latest smart watches track your body temperature, sleep quality, and can even detect electrodermal activity on your skin to gauge stress levels. | Editorial image credit: Kanut Photo / Shutterstock
Releasing heat and magnetic fields
Another intriguing development was the in-device treatment developed by the Hatton Cross team (comprising students from the University of Warwick, Imperial College London and Queen Mary University of London). The team is developing wearable technology that can detect wrist pain from sport, or the types of repetitive stress injuries arising from typing or writing too much.
One of the most fascinating aspects of the technology is the potential for in-device treatment. On the preventative side, the device could use vibration to alert users that their wrists are under strain. They also mentioned using heat from the device, or the release of a 0.05 Tesla magnetic field, to relax the muscles.
Another really insightful comment on the technology came from one of the judges. Dr Sarah Skerratt suggested that this type of technology - which is subtly attuned to the movements of the hand and wrist - could theoretically be used in the early diagnosis of Parkinson’s disease or Alzheimer’s disease. That is not to say there aren’t regulatory issues with developing wearable technologies for medical purposes, as the judges pointed out, but the potential of such devices is huge.
Wearable devices could be used to help diabetes sufferers, such as this Insulin Management System used by those with type 1 diabetes. | Editorial image credit: Maria Wan / Shutterstock
The staggering thing is that the technologies pitched by the Bright SCIdea finalists are just three of the myriad innovations being developed around the world at the moment.
Thirty years ago, few of us could have imagined that we would have a personal computer, music system, TV, watch, video, phone, camera, and games console all encapsulated within a single box that fits in our pockets. In 30 years’ time, we will scarcely be able to believe the health capabilities of the devices worn on our wrists and bodies.
Perhaps you will have heard of them first during the Bright SCIdea challenge?
What makes the Canada Awards so special, and which attributes do the winners share? We asked Bob Masterson, chair of SCI Canada’s Nominations Committee.
Bob Masterson, Chair, SCI Canada Nominations Committee
Why are the Canada Awards special to you?
The chemistry industry in Canada is an important industry – Canada’s third largest manufacturing sector with shipments of more than $80 billion (£48m approx.) a year. Behind that economic impact, however, are people. And, among those people are leaders.
The SCI Canada awards identifies both the lifetime leaders, as well as emerging student leaders in the business of chemistry. This serves to celebrate the achievements and inspire others in their pursuit of innovative chemistries.
What is so unique about the Canada Medal and what attributes have the previous winners had? Similarly, is there anything that binds the winners of these other prestigious awards?
The Canada Medal is unique in part due to its prosperity. It has been awarded since 1939. Looking at past Medal winners in aggregate, one can associate these individuals with being builders. Many individuals do good work in safely and efficiently operating their facilities. The Medal winners, however, are the builders.
They have attracted and deployed significant capital to build out the chemistry industry to ensure future prosperity for all Canadians. This is no small task in an industry dominated by global multinationals and very few truly domestic companies in Canada.
>> Find out more about the group and their awards on our SCI Canada Group page.
Would you mind explaining how the nominations committee comes to a decision on the award winners?
The Committee is made up of individuals with strong connections to industry and academia. They use their own experiences and solicit input from colleagues and other organisations to develop a list of potential candidates.
Committee members wishing to propose a candidate must prepare a short testimonial of why they have identified the candidate. The committee considers those testimonials while also looking for balance and diversity across industry and academia, Canada’s many regions, different types of chemistry, as well as representation across Canada’s highly diverse population.
The Canada Awards celebrate the best in Canadian chemistry.
Is there anything you’re particularly looking forward to in the pre-awards seminar?
The seminar gives us an opportunity to step back and reflect on the role and opportunity of chemistry as Canada transitions to be more sustainable. I look forward to hearing experts and people’s views on the important question of how we get there and what chemistry can contribute.
Why will it be so important to stage the awards in person this year (if possible)?
This year looks to be a special year. It will have been four years since SCI Canada last held an in-person Awards program. We all need some real time with real people. It’s long overdue and, for many, will be the first in-person event of any kind in over two years. I am sure there will be a lot of emotions.
The SCI Canada Awards 2022 will be held on 5 May 2022, in Toronto. Register your attendance on our event page.
>> Edited by Eoin Redahan. You can read more of his work here.
From learning what appeals to investors and increasing the public’s awareness of your products, there are huge benefits to be gained from winning competitions such as Bright SCIdea. So, how can you benefit from entering and what’s in store from this year’s shortlisted teams?
There was a fine article recently in Nature that crystallised the many benefits of entering science competitions, which extend far beyond the coveted prize money.
Winning the competition can take your product from obscurity into the eyes and minds of the public. Importantly, winning immediately gives your innovation credibility as your product (and your vision for it) will inevitably have been vetted by a team of expert judges.
You will also gain valuable publicity. Not only will the organisers promote these innovations, the new-found exposure will increase traffic to your own website and social channels.
Another really important facet of these competitions is that they help develop business sense in line with scientific innovation. In the aforementioned Nature piece, Ulrich Betz, Vice-president of Innovation at Merck, said: ‘Joining competitions can be a useful way for researcher-entrepreneurs to learn what appeals to investors and companies — training that many academic researchers lack… Participants have told me they’ve become more confident working in science and business after taking part.’
Indeed, this tallies with the experiences of last year’s BrightSCIdea winners, Metallogen. The team developed a novel nanoparticle spray that assists the natural process of phytoremediation to extract rare metals from mining. These metals can be sold on the market while decontaminating land next to mining sites at the same time.
Last year’s Bright SCIdea winners used a novel approach to boost metal recovery on old mining sites and decontaminate the land.
However, having an ingenious idea is one thing. Bringing it to market is another. And this is where the training for all the shortlisted teams helped. Metallogen’s John O’Sullivan and Rafael Hunt-Stokes said: ‘The competition has also taught us how to carry out market research and put together a cogent business plan, with the pitching training giving us the ability to convey our business idea in a compelling manner to investors and other stakeholders.’
>> Inspired by Metallogen’s success at Bright SCIdea? Read more about them in our news article.
This year’s Bright SCIdeas
So, from network building to training and advice on key areas such as intellectual property, these competitions can sharpen your innovations and bring them to that all-important next stage. That’s exactly what the shortlisted teams for this year’s BrightSCIdea plan to do.
This year’s entrants have certainly taken it upon themselves to tackle some of society’s grandest challenges. The Eolic Wall team, hailing all the way from the National University of Engineering in Peru and Universidade Estadual Paulista in Brazil, has created a wind energy system to help in our low-carbon energy transition. The Unmasked team (from the University of Durham) is also seeking to address the UK energy crisis while tackling waste by producing insulation materials from disposable face masks.
In health, the BioTech Inov (University of Coimbra, Portugal) team has entered a ‘highly efficient and versatile nanotechnological subcutaneous biomedical device with a high lifespan’, and the Hatton Cross team (from University of Warwick, QMUL, and Imperial College, London) has also submitted a wearable device that aims to enhance the wearer’s quality of life.
In an effort to address mental wellbeing, the Happy BioPatch team (from Oxford University and Manchester University) has created ‘a wearable gadget which continuously monitors cortisol levels aiming to prevent serious consequences as a result of stress’. Finally, the CardiaTec team (from the University of Cambridge) is specialising in tackling cardiovascular disease.
There’s so much to be gained from being part of competitions such as BrightSCIdea. We can’t wait to hear from the leaders of tomorrow.
Who knows? Maybe this will be the first you hear from a future Nobel prize winner?
>> Keep an eye out on Twitter for all of the wonderful innovations in this year’s BrightSCIdea competition at: @SCIupdate.
The clichés we use become so downtrodden that we often say them without thinking. How many times, for example, have you said you went with your gut on a certain decision?
As with many of these aphorisms, there appears to be genuine wisdom behind it. Scientists are learning all the time about the links between our guts and our brains, and recent findings from a California Institute of Technology-led (Caltech) study have added to our understanding of what’s going on behind our belly buttons.
This research contends that a particular molecule, produced by our gut bacteria, has contributed to anxious behaviour in mice. The Caltech researchers say that a small-molecule metabolite that lives in the mouse’s gut can travel up to the brain and alter the function of its cells. This adds further grist to the belief that there is a link between our microbiome, brain function, and mood.
The researchers behind the Nature paper say previous studies found that people with certain neurological conditions have different gut bacteria communities. Furthermore, studies in mice revealed that manipulating these communities can alter neurological states.
>> Curious about which herbs could boost your wellbeing and how they work in your body? Then read our recent blog on this topic.
Their study investigated the bacterial metabolite 4-ethylphenyl sulphate (4EPS) that is produced in the intestines of humans and mice and circulates throughout the body. In particular, they focused on the effect of 4EPS on mouse anxiety. For the sake of the study, mouse anxiety measured the creature’s behaviour in a new space - whether it hid in a new space as if from a predator or whether it was willing to sniff around and explore it.
The researchers compared two groups of lab mice: those colonised with pairs of bacteria that were genetically engineered to produce 4EPS, and a second group that was colonised with similar bacteria that couldn’t produce 4EPS. They then observed the rodents’ behaviour after being introduced to a new area.
Some mice become anxious when introduced to new spaces, and this is reflected both in the gut and the brain.
The results were very interesting indeed. The researchers observed that the group of mice with 4EPS spent far less time exploring this new place and more time hiding compared to the second group of non-4EPS mice. They also found that brain regions associated with fear and anxiety were more activated within this first group.
>> Interested in drug discovery? Why not attend our upcoming event at the Francis Crick Institute, London, UK.
When the mice were treated with a drug that could overpower the negative effects of 4EPS, their behaviour became less anxious. A similar study in Nature Medicine also found that mice were less anxious when treated with an oral drug that soaked up and removed 4EPS from their bodies.
The Caltech-led research could inform our understanding of anxiety and mood conditions.
‘It’s an exciting proof-of-concept finding that a specific microbial metabolite alters the activity of brain cells and complex behaviours in mice, but how this is happening remains unknown,’ says researcher Sarkis Mazmanian, in whose laboratory much of the research took place.
‘The basic framework for brain function includes integration of sensory and molecular cues from the periphery and even the environment. What we show here is similar in principle but with the discovery that the neuroactive molecule is of microbial origin. I believe this work has implications for human anxiety or other mood conditions.’
So, our predecessors were right: there’s a lot more to those gut feelings than you think.
>> Read the Nature paper on the Nature magazine website.
How well equipped is the UK’s battery supply chain to meet the growing demand for electric vehicles? We took a closer look to mark National Battery Day.
Main image editorial credit: Phaustov/Shutterstock
For many of us, it’s exciting to see the growth of the electric vehicle industry. Our personal transport will be cleaner. Our roads will be quieter. Indeed, from 2030 the UK government will ban the sale of pure internal combustion engine cars, and the widening role of ultra-low emission zones will hit many motorists in the pocket. Whether we like it or not, change is coming.
That does not mean we are prepared for it. As demand for electric and hybrid vehicles accelerates, and more stringent trade rules put pressure on having a local battery supply chain (stricter Rules of Origin for trade will come into force by 2027), the UK must get its complete supply chain up to speed.
Battery supply chain challenges
For this to happen, chemists, suppliers, manufacturers, innovators, government representatives, and others need to make strides in several areas. Over the past year, a group of more than 50 participants at SCI’s Energising the UK Battery Supply Chain workshops have identified next generation technology, the scale-up of innovative technologies, the skills and knowledge base, and standards for materials testing as areas for improvement.
Brine pools for lithium mining. There is a global clamour for raw materials including lithium.
The UK also needs a consistent stream of key battery materials. It needs technologies that reduce the dependence on some of the current materials for hybrid and electric vehicles. It must integrate efficient battery recycling and manufacturing approaches to reduce its dependence on long-distance imports and much coveted raw materials such as lithium, nickel and cobalt.
It is a big challenge. As David Bott, SCI’s Head of Innovation (who helped run SCI’s five Energising the UK Battery Supply Chain workshops) said, there isn’t enough of a UK electric battery supply chain at the moment.
>> Find out what the experts thought about improving the UK battery supply chain in our Energising the UK Battery Supply Chain Part 5 video.
David did note that the UK Government (through UK Research and Innovation) has been investing in the scale-up of cell assembly through the Energy Innovation Centre at WMG (from 2012/3) and the UK Battery Industrialisation Centre (through UKRI and the Automotive Propulsion Centre). It will also support the construction of Britishvolt’s electric battery ‘gigafactory’ in Blyth, Northumberland.
However, he added that: ‘All of them, however, are talking about the assembly of the cells and 60% of the value is in the materials. We need a battery materials supply chain in the UK – not all the way back to mining, of course, but as much as we can.’
Recent developments in the UK have been heartening, but many more will be needed to create a viable battery supply chain.
Smoother collaboration is also required. ‘We need recognition that the UK needs more support for the chemistry part of the supply chain,’ he said. ‘We need a lot more collaboration – engineers need to understand that chemistry companies would engage more if they understood the size of the opportunity. The main thing we need at the moment is awareness of the opportunities.’
Promising developments
Despite the difficulties, green shoots have appeared recently. In late January, the government announced that it has backed Britishvolt’s aforementioned plans to build large volumes of electric vehicle batteries (through the Automotive Transformation Fund). According to the government, the factory will produce enough batteries for more than 300,000 vehicles a year and create 3,000 direct, highly-skilled skilled jobs. Britishvolt have also announced a partnership with Glencore to recycle battery materials.
>> Sign up for our next Energising the UK’s Battery Supply Chain workshop.
Oxford-based chemical products manufacturer Nexeon has secured US$80 million (about £59 million) in funding to scale up the production of its silicon anode materials. Finally, Sheffield-based sodium-ion battery technology company Faradion has been acquired by Indian conglomerate Reliance Industries for £100 million. A further £25 million will be invested as growth capital to accelerate the commercial rollout of its sodium-ion battery technology.
Faradion says that its sodium-ion technology provides ‘significant advantages compared to lithium-ion technology, including greater sustainability, a patented zero-volt safe transport and storage capability’.
So, there is some good news to celebrate as you gather around with your families to celebrate National Battery Day. The battery supply chain, unfortunately, must wait for another day.
What is the future of electric cars? Find out more in this Autotrader article.
Machine-made snow has made this Winter Olympics happen in Beijing, but at what cost?
If you take a look at the weather in Beijing right now, you’ll notice that it isn’t really that cold. You can enjoy daily highs of about 8°C in early February, which we’d be happy enough here in London.
These mild conditions have been a problem for the organisers of the Winter Olympics, which are currently taking place in Beijing and environs. Indeed, the distinct dearth of snow has meant that the Beijing Games have become the first to be run largely on artificial snow.
Snowmaking machines spray artificial snow on a ski slope during the FIS Ski Cross World Cup, a test event for the 2022 Winter Olympics
For some, the presence of 130 fan-driven snow generators and 300 snow-making guns spewing out machine-made snow represents a waste of resources, even if these machines are powered entirely by renewable energy.
In all, 49 million gallons of water will reportedly be used to make the Games possible. So, to say they are water-intensive is something of an understatement. However, the issues don’t end there. There is also an issue with the type of snow produced.
>> What can you do about climate change? Register for this free talk to find out more.
Some claim artificial snow creates more dangerous conditions for athletes.
According to the recent Slippery Slopes report written by the Sport Ecology Group (in conjunction with Loughborough University UK and Protect Our Winters UK), the composition of artificial snow can create dangerous conditions for the athletes. Basically, it creates a faster, harder surface that could result in more severe injuries.
The reason given for this is that artificial snow is almost 30% ice and 70% air, compared to natural snow, which is closer to 10% ice and 90% air. This ‘grittier ice-pack’ creates tougher conditions for athletes, many of whom travel at great speeds down steep slopes.
In the same report, former Winter Olympian Laura Donaldson explains why these machines create suboptimal snow. ‘The artificial snowflakes they generate have cylindrical structures (unlike the far more intricate structure of natural flakes),’ she said, ‘which mould together to form bulletproof ice conditions.’
Furthermore, this less permeable layer of ice may hinder the growth of vegetation, and the noise of the machines disrupts wildlife. In some resorts, chemicals are also added to create longer lasting snow.
At Beijing, the organisers claim not to have used chemicals in the snow-making process. However, others rely on machines and chemical-kind for a helping hand. According to the Sport Ecology Group report, a pesticide was used at the 2010 Games in Vancouver to allow the water to freeze at higher temperatures; and snow hardeners such as salt and fertiliser have been used to improve snow quality on cross-country skiing trails.
If hosting the Winter Olympics in an area without much snow seems crazy to you, it might not be quite as daft as you think. The bleak reality is that global warming is reducing the number of venues that can host this enormous event without artificial help.
According to an academic paper by Scott et. al. in 2014, only six of the last 19 Winter Olympics host cities will still have the climatic conditions to do so by the 2080s. Of course, that doesn’t take artificial snow into account.
So, when you see Qatar being awarded the 2050 Winter Games, don’t tell me you haven’t been warned.
How do you create an investor-ready intellectual property (IP) approach to help you secure that all-important funding? We asked Charlotte Crowhurst, patent attorney at leading European IP firm, Potter Clarkson.
As businesses focus on growth in the post-pandemic world, innovation is vital. Being able to turn good ideas into a commercial success – at scale – can have a transformational impact on the wider economy. Scientists and engineers have been front and centre in providing solutions to the health crisis, but they will also play an essential role in the economic recovery.
Of course, even the most ground-breaking invention requires investment to become a viable market proposition. Yet, the road to securing funding is not always straightforward or clear, with various hurdles to overcome before winning the trust and backing of investors. Securing funding is fiercely competitive territory, as investors apply a forensic approach to identifying the risks and opportunities with each investment target.
Intellectual property alone will not likely secure funding, but a weak IP position could significantly impact on valuation – by as much as 70% – or even see an investor walk away altogether. What’s more, for return-hungry investors, new research shows that SMEs with intellectual property rights generate 68% higher revenues per employee than those who don’t.
For ambitious, high growth SMEs to put themselves in the strongest position to attract and secure funding, there are five key ingredients that make up an investor-ready IP approach:
- Clear ownership
This is the number one deal breaker. Make sure there are no grey areas on ownership of IP. Any grey areas surrounding who ‘owns’ IP will signal alarm bells for a potential investor.
- Effective innovation capture
Understanding what IP your business may have and what you might be able to protect is not always obvious. It is always worth seeking professional advice early on to determine which IP rights you might be able to secure.
Robust processes and procedures are also important. Create an IP register and keep it up to date monthly so that opportunities are not overlooked. Do not underestimate the importance of robust processes and procedures.
Understanding what IP you need to protect isn’t always obvious.
- Sound strategy
Put yourself in an investor’s shoes – they are focused on whether you can provide a return on their investment. They are looking for clarity in your approach – a strategically sound business plan, where it is easy to see how the IP rights will help to achieve the commercial objectives.
>> Need more information on filing a chemistry patent. Read our blog on chemistry patent filing.
- Market awareness
A growing business can be all-consuming, but a sound IP approach takes into consideration the wider marketplace in which your business is operating and any potential third-party rights.
- Good timing
Knowing when to act is critical to a sound IP approach. Knowing which steps to take and when to take them can have a critical impact on the strength of your IP position.
The end goal
Ultimately, the end goal with IP due diligence is to instil confidence and build trust with a potential investor. While investors are prepared to take on varying degrees of risk, SMEs will always need to show an IP approach that doesn’t signal alarm bells.
Put simply, those SMEs who are clear on these five areas will reduce the chances of IP being the reason an investor walks away.
>> To read more on ensuring your IP is investor-ready, visit the Potter Clarkson website here.
Edited by Eoin Redahan. You can find more of his work here.
The plant-based meat alternative market is growing rapidly, and cell-cultured meats could be coming soon to your dinner plate once they receive regulatory approval. Gavin Dundas, Patent Attorney at Reddie & Grose, provides his expert perspective on the state of the meat alternative market.
Which is receiving more emphasis based on patent activity: lab-grown meat or plant-based meat alternatives?
Comparing cultivated meat to plant-based meat is a bit like comparing apples and oranges.
Plant-based meat is here - it’s in shops, and it’s in growing numbers of restaurants and fast-food outlets. Even McDonald’s – arguably the world’s most well-known hamburger outlet – released its first plant-based burger in the UK on 13 October 2021: the aptly-named McPlant. The McPlant has been accredited as vegan by the Vegetarian Society, and includes vegan sauce, vegan cheese and a plant-based burger co-developed with Beyond Meat.
Cell-cultured meat is a very different prospect, as cellular agriculture is more high-tech, so companies entering that sector require a higher degree of specialised technical expertise. Companies delving into cultivated meat also require a fair bit of funding, as cultivated meat has not been approved for sale in any country other than Singapore, so it is not yet possible to sell their products to consumers.
The reality at the moment is that plant-based meat alternatives have a huge head-start in the marketplace, while cultivated meat is not yet on sale in most countries. So, for most new companies looking to make money in the alternative protein market, plant-based products are likely to be the easier way to start.
On the other hand, this means that the plant-based meat market is more crowded already, while cultivated meat companies are investing in the hope of getting a bigger share of that market once it matures.
In which food types have you seen a particular surge in patent applications, for example plant-based meat alternatives or lab-grown meat?
Based on searches using patent classification codes commonly used for plant-based meats and lab-grown meat (known as ‘cell-cultured meat’ or ‘cultivated meat’), it appears that there are significantly more patent applications in the field of plant-based meats, but that patent filings relating to cultivated meat are growing more quickly.
Of all the patent publications relating to plant-based meats, 15.2% were published since the start of 2020. Of the patent publications relating to cultivated meats, 27.6% were published since the start of 2020.
This outcome is probably not surprising. Plant-based meats have been around much longer and are now widely established in the market, so many more companies have had time and opportunity to file patent applications for innovations in this area. Cultivated meats are at an earlier stage in their development, but with a large number of new companies having been formed in this area in the last few years, it is not surprising that this has resulted in a high growth rate of patent applications as cultivated meat gets closer to commercial reality.
Beyond Meat’s plant-based meat substitutes have reached the mainstream. | Jonathan Weiss/Shutterstock
How much movement has there been on the equipment and other innovations that will facilitate large-scale meal alternative manufacturing?
There is a huge difference between small-scale production of cultivated meat in a laboratory, and the large-scale manufacturing that would be needed to supply supermarkets and restaurants throughout whole countries and - eventually - the whole world.
Growing meat using cellular agriculture involves the use of animal cell lines to grow animal products in bioreactors, where the cells are immersed in a growth medium that feeds nutrients to the cells as they develop. Over the last decade there have been huge advances in these processes, but as demand for cultivated meat grows there will definitely be continued innovation to improve efficiency and scale-up manufacturing capacity.
Commercial growth medium is currently costly, so the development of more cost-effective growth media is likely to be an area of much research. Another ongoing challenge is the development of high-quality cell lines and scaffold materials that are suitable for high-quality, large-scale production.
Bioreactor design is also expected to be a big area of innovation - up until now, bench-top bioreactors have in most cases been sufficient to meet the demands of cultivated meat R&D, but as demand increases bigger and better bioreactors will be needed. A particular challenge will be to design bioreactors capable of growing thick tissue layers on a commercially viable scale.
While there is scope for innovation in all of these areas, some companies are already ready to manufacture their cultivated meat products on a large scale. Future Meat Technologies, for example, opened its first industrial cultivated meat production facility in June 2021 in Rehovot, Israel - that facility is reportedly capable of producing 500kg of cultivated meat products every day. In November 2021, Upside Foods opened its first large-scale cultivated meat production plant in Emeryville, California, with the capacity to produce 22,680kg of cultured meat annually.
At the moment, however, a lack of regulatory approval is holding back cultivated meat production. While there are a number of companies that apparently have products ready for market, many will be unwilling to plough huge amounts of money into large-scale manufacturing facilities until they have regulatory approval that lets them actually sell their products.
Thinking of filing a chemistry patent in 2022? Here’s what you need to know.
The UK has cutting-edge companies in the cultivated meat field.
Have any innovations or areas of innovation struck you as particularly exciting? If so, could you tell us more about them?
I am a meat-eater trying to cut down on my consumption of meat, due to a mixture of environmental and ethical motivations. So, as a consumer I’ve been very excited to see the arrival of plant-based meat into the mainstream.
I am particularly excited to try cultivated meat once it is approved for sale. Not long ago ‘lab-grown’ meat seemed like science-fiction, so to get to a point where you can go out and buy it will be incredible. So many people are unwilling to cut down on meat because they like the taste, and because their favourite meals are meat-based, so cultivated meat might hopefully give that same experience with fewer of the drawbacks of animal meat.
I am also excited to see the diversity of cultivated meat products. Cultivated meat chicken nuggets and beef burgers are the products that spring to mind when cell-cultured meat is mentioned, but there are companies out there developing cultivated bacon, pork belly, salmon and tuna, to name a few.
What are the chemistry challenges for those creating plant-based meat alternatives? Find out here.
Given what you know about the patent landscape, where do you think the meat alternative industry is heading, and at what sort of pace do you foresee significant change?
I think the meat alternative industry is only going to continue to grow, as concern over the environmental impact of our eating habits is growing, and the quality and availability of meat alternatives is getting better.
The plant-based meat industry is already doing well, and I expect it to continue on its upward trajectory. I expect companies in this field to continue to file patent applications for their innovations, and eventually we might see some of those patents being enforced to safeguard valuable market shares for the patent owners.
Cultivated meat is the sector that seems to be poised for the most significant change. At the moment, the lack of regulatory approval seems to be the thing holding it back, but if that hurdle is removed there are UK companies aiming to get cultivated meats into shops by 2023. The UK is lucky enough to be home to a number of cutting-edge companies in the field, and a recent report by Oxford Economics researchers forecast that cultivated meat could be worth £2.1 billion to the UK economy by 2030.
The idea of cultivated meat is unlikely to appeal to everyone, so I imagine that it will start out as something of a novelty, but I’d expect to see the availability and range of cultivated meat products grow significantly over the next decade.
Edited by Eoin Redahan. You can read more of his work here.
Are you thinking of filing a chemical industry patent in 2022? Anthony Ball, Senior Associate at patent attorney Abel + Imray, gave us the lowdown about what you need to know about the process, cost, and filing your patents in different countries.
I’ve developed a novel technology. How do I patent it, how long does it take, and how much could it cost?
The first step in patenting a novel technology is to file a patent application. The patent application must contain a description of the technology that you have developed in enough detail for others to work the invention. It also needs to contain some claims that define the protection you think you are entitled to. Before the application is filed, it is also important to sort out who the inventors are and who owns the invention.
The application is then examined, during which the Patent Office and you come to an agreement regarding the extent of protection that you are entitled to. Once the extent of protection is agreed, the patent will proceed to grant.
The application will be published around 18 months from filing. This allows competitors to see what you intend to protect. It usually takes longer for the patent to be granted (and so be enforceable) - usually from four to 10 years. For a UK patent which protects a chemical invention, the total cost might be around £10,000.
A separate patent is required for each country that you are likely to want to stop competitors using your technology. Obtaining patents in the most important markets might cost in excess of £50,000 for a chemical invention. Although this might sound like a lot of money, not all of this needs to be paid at the start of the process. Instead, it is spread out over a few years, with the biggest investment usually coming three years into the process.
You mentioned that you can obtain a patent for a compound, a formulation, or a process for synthesising compounds. Does the patent process and cost vary according to the type of product or the branch of chemistry?
The overall process – filing a patent application, the patent application being examined and then the patent being granted – is the same for all technologies. However, there are some issues faced in certain branches of chemistry (such as pharmaceuticals) which can be quite difficult to overcome, and are not faced as commonly in other branches of chemistry. Because of this, it can sometimes take longer for patents in these fields to be granted than in other fields of chemistry, and the costs can be higher.
In which scientific areas has there been a recent rise in patent applications and are any fields relatively under-represented by comparison?
Focusing on European Patent Applications, the chemical industry has been fairly strong recently. Pharmaceutical and biotechnology in particular saw relatively large increases in the number of European patent applications filed in 2020, although the number of patents in the organic fine chemical field slightly decreased.
I want to file my patent in several countries. What do I do, and how much do the costs vary, depending on the country? For example, how would the cost of a patent in the UK compare to one in the US?
If you wish to have a patent in several countries, the start of the process is the same as the one described earlier; a patent application is filed in one country. Then, the most cost effective way to extend the protection to other countries is usually to file a “PCT application” within a year of filing the original application. After a further 18 months, you can turn this PCT application into applications for most countries around the world, including Europe, the US, China and India.
Costs do vary between different countries. To use the example above, it might cost 50-100% more to obtain a patent in the US than in the UK alone. It is worth noting that a patent for the same technology from the European Patent Office might cost around the same as a patent in the US, but the patent from the European Patent Office can then be converted into a patent in each country in the EU, plus some others (including the UK, Norway and Switzerland). Unfortunately, it is difficult to be precise about costs, because they depend very much on the number and type of objections raised by the patent office examiners.
One other consideration is translations. For long applications (which can be quite common in some branches of chemistry), these can be expensive, adding thousands of pounds to the cost for obtaining a patent. One country in particular where a translation might be required, and is of growing importance in the chemical area, is China.
Patents from the European Patent Office are valid across the EU and in several other countries. | Editorial credit: nitpicker / Shutterstock.com
>> From patents to green chemistry and agrifood, we have some great events coming up. Find out more on our event page.
Is there anything chemists and chemistry industry professionals should be particularly mindful of when submitting patent applications in 2022?
Patent law is underpinned by a number of international agreements, which are hard to renegotiate. As a result, the law is actually very stable over time, and so the considerations in 2022 will broadly be the same as they have been in the past. Having said that, one important thing to bear in mind at the moment is the amount of data to include in the patent application.
There is a balance between filing as soon as possible (to prevent a competitor getting there first, and to minimise the chance of a disclosure of something that would make your technology unpatentable), and making sure that the application has enough data to show that the extent of protection that you are asking for is justified. In some cases, it is possible to present data to justify the scope of protection after the application has been filed, but recently many patent offices have made that more and more difficult.
As such, filing too early, and with only a small amount of data to support your claims, could result in a very narrow patent, which might potentially be easy to work around. It is very important to include enough evidence to show that at least the parts of your invention which have the most commercial interest (e.g. the most active compounds) show the technical effect which is mentioned in the patent application.
How much have the law and process around patents changed in recent years?
The law around patents and patent applications is always evolving, albeit slowly. The basics – that the technology must be new, not be obvious in view of publicly available knowledge, and have an industrial application – have remained the same for many years. Likewise, the basic process to obtain a patent, as described above, has not changed recently, but the minor details of that process are constantly being updated, for example to incorporate new technology (such as online filing of the application and supporting documents, and online publication of the application) and to improve cooperation between the patent systems of different countries.
An example of improved cooperation between countries is the Unified Patent Court (UPC), which is likely to begin hearing cases in 2022. Currently, patents have to be enforced in each EU country separately using the national court systems. The UPC will establish a common court system and allow a patent to be enforced in one court case, with the result being valid for the whole of the EU.
I have made a further development to my technology after filing my patent application. How can I protect my new development?
Once it has been filed, nothing can be added to a patent application. Because of this, if you want to protect a new development to the technology that is the subject of a patent application, then another patent application must be filed directed to the new development. The two applications will be treated separately, and so in order for a patent to be granted which protects the new development, the new development must satisfy all the criteria for patentability described above.
To read more from Abel + Imray on patents, visit: https://www.abelimray.com/
At COP26, Nikita Patel co-hosted the Next-Gen debate, where an inspiring group of young people discussed how chemistry is tackling climate change. The PhD student at Queen Mary University of London shares her experience.
While the United Nations Climate Change Conference (COP26) may be over, there is still plenty to be done in the fight against climate change. We’ve seen what can be achieved when we work together and no doubt science will play a key role.
On Thursday 4 November, I had the privilege of co-hosting the Countdown to Planet Zero Next-Gen debate organised by SCI to showcase the work being carried out by our young and innovative scientists to tackle climate change. It was a real pleasure to share the stage and hear from some great scientists, exploring the themes Fuels of the Future, Turning Waste into Gold and Engineering Nature. The event gave the audience the opportunity to question and challenge the panel members on their climate change solutions.
Panel L-R: Dominic Smith, Natasha Boulding, Clare Rodseth, Jake Coole, Nikita Patel, Oliver Ring (Brett Parkinson joined virtually).
While I was feeling nervous about my hosting duties, I was very excited at the same time as I knew how important it was to educate the audience, whether they were members of the public or aspiring scientists, on how science is crucial in battling the climate emergency.
An important part of my role as a host was to ensure the incoming questions and comments were understood by all, given the mixed audience attending. This highlighted how essential good science communication is to prevent misunderstandings and the spread of misinformation.
It was brilliant to see how engaged the audience were from the flurry of questions that came in during the session, so much so that we didn’t manage to get through all of them! There were a wide variety of questions aimed at particular panellists but also towards the panel as a whole. It was thought-provoking to hear how scientists from different backgrounds offered their own perspectives on the same topic.
4 November was also Energy Day at COP26 and the atmosphere was buzzing! I learnt a lot from attending the Green Zone, not only from our panellists but from all the exhibitors present too. I appreciate the small, individual actions we can each take that will make a difference but also the need to work together to achieve the common goal of fighting climate change. It was clear to see how science and business go hand in hand to provide solutions to society and how interdisciplinary collaboration is key.
The result of our poll question: ‘Do you think that science is pivotal in providing climate change solutions?’ spoke for itself, with a resounding yes from 100% of the audience participants! This was a very positive outcome and showed that it is not all doom and gloom when it comes to discussing the climate crisis.
On a personal level, I'm going to continue implementing some simple changes like using public transport more, eating more vegan food and flying less and aim to keep the discussion going with my peers as the climate emergency is far from over.
SCI team, panellists and hosts.
I hope the youth panel event has inspired the next generation of scientists and showcased some of the exciting work that is going on behind the scenes which people may not realise and ultimately, that there is hope in science.
>> To rewatch the event, the recording is available on the COP26 YouTube channel: Countdown to Planet Zero Combating climate change with chemistry | #COP26, and on our Climate Change Solutions hub.
>> Want to read more about the technologies discussed by our panel? Read our event review: https://www.soci.org/blog/2021/11/2021-11-05-cop26-review.
‘This is a fragile win. We have kept 1.5 alive. That was our overarching objective when we set off on this journey two years ago, taking the role of the COP presidency-designate. But I would say the pulse of 1.5 is weak’ – Alok Sharma, President for COP26.
If scientists, politicians and activists were hoping that COP26, delayed by one year because of the pandemic, would yield concrete plans for progress on climate change, perhaps the overall conclusion might be ‘at least we haven’t gone backwards’.
The Glasgow Climate Pact, signed by 197 countries, required an extra day of negotiations. In his summing up, the UN Secretary General António Guterres said: ‘The approved texts are a compromise. They reflect the interests, the contradictions, and the state of political will in the world today.’
In his video statement Guterres said that the agreement ‘takes important steps but unfortunately the collective political will was not enough to overcome some deep contradictions. We must accelerate action to keep the 1.5 (degrees °C) goal alive…it’s time to go into emergency mode or our chance of reaching net-zero will indeed be zero.’
Guterres added that it was his conviction that it was time to phase out coal, end fossil fuel subsidies and build resilience in vulnerable communities. He also addressed the many young people and indigenous communities, saying: ‘I know you are disappointed. But the path to progress is not always a straight line…but I know we will get there. We are in the fight of our lives, and this fight must be won.’
COP26 President Alok Sharma believes that the measures agreed at COP26 are a ‘fragile win’ in the fight against catastrophic climate change. | Editorial credit: Paul Adepoju / Shutterstock.com
The Glasgow Climate Pact calls on signatories to report their progress towards more climate ambition in time for COP27, which will be hosted by Egypt. Welcoming the agreement, Alok Sharma, COP26 President, said: ‘This is a fragile win. We have kept 1.5 alive. That was our overarching objective when we set off on this journey two years ago, taking the role of the COP presidency-designate. But I would say the pulse of 1.5 is weak.’
European Commission President Ursula von der Leyen said: ‘We have made progress on three of the objectives we set at the start of COP26. First, to get commitments to cut emissions to keep within reach the global warming limit of 1.5 degrees. Second, to reach the target of $100 billion per year of climate finance to developing and vulnerable countries. And third, to get agreement on the Paris rulebook. This gives us confidence that we can provide a safe and prosperous space for humanity on this planet.’
The NGO Greenpeace said in a statement: ‘While the COP26 deal doesn’t put the 1.5C goal completely out of reach, the governments and companies that obstructed bold action on climate change are knowingly endangering whole communities and cultures for their own short-term profits or political convenience. History won’t judge them kindly for this.’
While the final Pact has not reflected the hopes of many, it can be said that COP26 wasn’t short of a desire to see change. Perhaps the surprise package of the two-week event was the declaration between China and US which states that the countries ‘…recognise the seriousness and urgency of the climate crisis. They are committed to tackling it through their respective accelerated actions in the critical decade of the 2020s, as well as through cooperation in multilateral processes, including the UNFCCC process to avoid catastrophic impacts.’ The declaration from the two countries was widely welcomed.
Methane emissions and ocean protection
Other notable developments from COP26 included: The formal launch of the Global Methane Pledge led by the US and the European Union. The Pledge, which seeks to reduce overall methane emissions by 30% below 2020 levels by 2030, saw 100 countries, representing 70% of the global economy and nearly half the global methane emissions, sign up.
In agriculture, the Agriculture Innovation Mission for Climate (AIM4Climate) was launched. Initiated by the US and United Arab Emirates, with endorsement from the COP26 Presidency, the goal of the initiative is to increase and accelerate global innovative research and development on agriculture and food systems in support of climate action.
For some, including environmental activist Greta Thunberg, the resolutions agreed by governments at COP26 are insufficient. | Editorial credit: Mauro Ujetto / Shutterstock.com
The initiative has the backing of 32 countries. In addition, ocean protection received a boost with the UK Government using the COP26 Ocean Action Day to announce a wave of investment including at least £20 million in commitments made at the Ocean Risk and Resilience Action Roundtable to drive the health and resilience of the oceans and climate vulnerable communities.
The Science and Innovation day at COP26 saw the launch of four initiatives, backed by global coalitions of nations, businesses and scientists. In what was said to be a global first, the Adaptation and Research Alliance was launched. The network of more than 90 organisations will collaborate to increase the resilience of vulnerable communities most impacted by climate change.
In further developments the UK, along with several countries including Canada and India, will collaborate to develop new markets for low carbon steel and concrete. The work is being carried out under the Industrial Deep Decarbonisation Initiative.
The need for innovation
Commenting on this, George Freeman, the UK Minister for Science, Research and Innovation, said: ‘Real change to combat climate change cannot happen without new scientific ideas, innovation and research, and it is clear no country or company acting in isolation can deliver the change that is needed at the pace that is needed.’
While the final COP26 Glasgow Climate Pact has disappointed many, there is no doubt that there is a will to make positive change, keep global temperatures in check and see humanity reap benefits.
How do you get large audiences to read about your work? Roger Highfield, Science Director of the Science Museum, and Steve Scott, Public Engagement Lead of UK Research and Innovation, shared their insights at a recent webinar organised by SCI.
‘When I talk to people about science writing – when I’m talking about the introduction – I ask them to practise on a long-suffering friend and read a couple of paragraphs of what they’ve written. If they reach for their phone, you’ve done something wrong.’
Some people’s observations should be taken with a liberal fistful of salt, but Roger Highfield is certainly worth listening to when it comes to connecting with the public. As Science Director of the Science Museum Group, he helped engage with more than five million visitors in 2019/20 alone and has written and edited thousands of articles as Science Editor of the Daily Telegraph and Editor of New Scientist.
Roger Highfield, Science Director of the Science Museum
So, how can you reach large audiences with scientific content? First of all, salience is important. How does what you’re talking about have a material effect on people’s lives? As Roger Highfield noted dryly: ‘People will be very interested in asteroids when one’s bearing down on the Earth.’
Citizen science and the long form Q&A
Similarly, the public has been voracious in its consumption of Covid-19-related content despite the complicated nature of the virus and vaccine development. During lockdown, Roger Highfield’s long form Q&A blogs about Covid-19 were hugely popular because, as he said, ‘there was a public appetite for a deeper dive into the science’.
Aside from writing in a way that decongests heavy, complicated subjects, it also helps to get your research in front of the right people, namely communications specialists. ‘One lesson for mass engagement is to work with media organisations,’ he added. ‘It’s more than a platform – you’re dealing with experts in public engagement.’
For larger organisations, citizen science is an excellent way to engage people by making them part of a project. The Great Backyard Bird Count is a fine example of citizen science at its simple, effective best, with thousands of bird-watchers helping provide a real-time snapshot of bird populations around the world.
Highfield has engaged with the public in all manner of citizen science initiatives, from recent online cognition tests in which 110,000 people took part, all the way back to an experiment asking people about the catchiest song in the world. ‘At the time, it was The Spice Girls’ Wannabe,’ he said. ‘People recognised it in 2.5 seconds.’
At its best, citizen science doesn’t just help you to engage people in your work; it can be used as a valuable way to gather information and provide unique perspectives. ‘Citizen science is not just a flash in the pan. The role is changing,’ said Steve Scott, Public Engagement Lead at UK Research and Innovation (UKRI). ‘It’s an effective way of gaining knowledge… bringing different forms of knowledge and expertise into research.’
Steve Scott, Public Engagement Lead of UK Research and Innovation
Scott used the University of West London-led Homes Under the Microscope project to illustrate his point. As part of this project, people in Bristol and Bradford will detect and monitor airborne microplastic sources in their homes and feed this information back to the project organisers to help assess the prevalence of these substances.
A cultural shift
If you’d like more people to read about your research or product, it’s also worth thinking about the way people consume media. According to Scott, the general public tends to consume science through televisions and museums (for example, a visit to the zoo), and people are most likely to follow up on scientific matters having seen them on the news.
Many people learn about science through social media and YouTube, but other vehicles are worth considering too if you want to raise awareness. The UKRI views gaming as a significantly untapped area of public engagement and is investing in this area. Another intriguing way to raise awareness of innovative research is through awards, with the recent, well publicised Earthshot Awards providing a case in point. ‘They’ve taken research grants,’ Scott said, ‘and made them into the Oscars.’
Encouragingly, as the means of communication are changing, so too is the readiness of researchers to share their work. Both Highfield and Scott have seen a large shift over the past 15 years or so, with more and more scientists communicating their research. ‘It’s recognised as being an important part of being a researcher now,’ Scott said. ‘You’re excited about [your research]… Why would you not talk to the public about it?
The big takeaway
So, what is the most important takeaway from the talks, apart from that all-important Spice Girls fact? Fundamentally, when you are communicating your research or peddling your company’s wares, it helps to narrow your focus.
Indeed, Scott reminded us that the public is not a homogeneous group. ‘If we want to engage with millions of people, we need to think of audiences as more than just the general public,’ he said.
He said that 75 per cent of the potential UK audience – roughly 49 million people – falls into one of two groups: they don’t think science is for them, or they’re inactive. So, it’s worth taking an in-depth look at your target demographic and the places it goes to for news before sharing your work.
Earlier, Roger Highfield emphasised the same thing. He said: ‘If there’s one thing I want you to take from this talk, it’s to think about the audience.’
>> Watch How to engage with millions of people in full on our YouTube channel at: https://youtu.be/HSOMQd958EQ
Continuing our profiles of Black scientists, Dr Jeraime Griffith, Chair of SCI’s Agrisciences Group, shares how a simple classroom experiment set him on the journey that has led to him analysing complex data to safeguard UK food security.

I am a Data Scientist building tools that maintain, forecast and predict threats to the UK’s food security.
Right: Dr Jeraime Griffith
What was it that led you to study chemistry/science and ultimately develop a career in this field? Was this your first choice?
At about age 10, in primary school, I had a teacher who explained to us how the human digestive system and saliva break down starch into sugars. To demonstrate this, he got some bread from the school kitchen and asked us to chew it until we started noticing a slight sweet taste. I decided then to be a scientist. This wasn’t my first choice however. Prior to that moment, I wanted to be a pilot.
Was there any one person or group of people who you felt had a specific impact on your decision to pursue the career you are in?
My parents were super supportive. After announcing that I wanted to be a scientist, I got a science dictionary for my birthday. I also had great teachers, both at primary and secondary school. At 13, we were doing hands-on chemistry experiments and helping to tidy the lab at the end of the school year.
Could you outline the route that you took to get to where you are now, and how you were supported?
Following a BSc and a PhD, both in chemistry, I worked for ChemOvation, Argenta Discovery (now part of Charles River Laboratories) and briefly at Novartis. I then went off to New Zealand for a two-year postdoc at Massey University in early 2009 to work with my former PhD supervisor who had relocated there.
On returning to the UK, I worked at Imperial College London, first at the Centre for Synthetic Biology, then over in Chemistry with Professor Tom Welton. It was towards the end of my time with Professor Welton that I began learning the programming language Python, which led me to data science. I’m now a Data Scientist at Cognizant, working with the Food Standards Agency.
I was fully supported, both in industry and academia, but it was in academia that I was afforded the freedom to explore my interests – particularly to use 20% of my time to do whatever I wanted.
Jeraime helps safeguard UK food security and Chairs SCI’s Agrisciences groupConsidering your own career route, what message do you have for Black people who would like to follow in your footsteps?
Allow some flexibility in pursuing your career. When I was questioning myself and my goals, I came across ‘Obliquity’, a book by John Kay. Sometimes diversions are the best way to get to your goals.
Seek out mentors, and I would say regardless of race, who can help you get there. Don’t be afraid to email them and briefly talk about your interest in the work they’ve done, what you have done and are doing now. I’ve found people are genuinely interested in helping you. This is how I learned about the Agrisciences group at the Society for Chemical Industry, which I joined and now Chair.
As for getting into data science, I did a 13-week intensive bootcamp. These are not for everyone as they are expensive and have a high demand on your time. However, there are a lot of free courses available. With this availability, it can be hard to find the good ones. The knowledge of the crowd can help. I’ve found Twitter to be our modern day equivalent to Ask Jeeves.*
What do you think are the specific barriers that might be preventing young Black people from pursuing chemistry/science?
Lack of representation I think is the number one barrier. Impostor syndrome is bad at the best of times, but worse still if there’s no representation in the ivory tower.
What steps do you think can be taken by academia and businesses to increase the number of Black people studying and pursuing chemistry/science as a career?
Recruit people of colour with less experience to positions of responsibility. Trust us to perform and have the support in place when we falter.
The experience that most defined Jeraime’s career path… a great teacher
Science is at the centre of addressing many of the big global issues. Do you hope that this will lead to more young Black people wanting to get involved in science and develop solutions?
Yes. A low entry point is data science. Most of the tools we use are open source. Data for your area of interest are, for the most part, freely available and the data science community is helpful and engaging.
Could you share one experience which has helped to define your career path?
Where I am now began in that class in primary school when I first learned about the human digestive system. So, my defining experience would be having a great teacher.
*Note from the editor: Some youngsters may need to look up what Ask Jeeves is!
Edited by Muriel Cozier. You can read more of her work here.
As we build up to the 3rd SCI-RSC symposium on antimicrobial drug discovery, we spoke to Dr Anita Shukla, Associate Professor of Engineering at Brown University, about designing drug delivery systems to treat infection, creating a positive atmosphere in her lab, the challenges facing professionals in her industry, and much more.
Anita Shukla, Associate Professor of Engineering at Brown University
Tell us a bit more about the work being done in your lab.
All of what my lab works on is very biomedically orientated. The major thing we focus on is treating bacterial and fungal infections. We have a lot of interest in designing drug delivery systems to treat all sorts of bacterial and fungal infections, from localised infections to more systemic infections. We design nanoparticles, polymeric nanoparticles, self-assembled structures, surface coatings and larger-scale materials such as hydrogels that can be used as bandages.
We work on the material design for delivering antimicrobial therapeutics – antibiotics, antifungals and other antimicrobial components – and we study a lot about the properties of these materials. What sets us apart is that we’re trying to make materials that are smart, that are in some way targeted or responsive to the presence of bacteria or fungi.
So, to give you an example, we are working on making hydrogel wound dressings. These wound dressings are smart and can respond to the presence of bacteria and fungus. They know when bacteria and fungi are present, based on the enzymes that are there in the localised local environment of the hydrogel. They actually degrade only in the presence of those enzymes and release encapsulated nanotherapeutics.
And that’s really important because of antimicrobial resistance. So, we are trying very hard to provide effective therapies but limit exposure to antimicrobial therapeutics only to times that they’re needed. That’s the kind of work we’ve been doing over the past five or six years.
You’ve done some really interesting work on pregnancy care too. Tell us more about that.
So, that work was inspired by a graduate student who was very interested in women’s health and prenatal health. What we noted was that a lot of pharmaceutical agents that you must use when you’re pregnant don’t have enough information associated with their potential toxic side effects on a growing fetus. A lot of that testing is very difficult to do, so we thought: ‘Can we come up with model systems that could be used for the testing of pharmaceutical agents, toxins, and toxicants?’
The placenta really is the interface between the fetus and the mother and a lot of the nutrient and waste exchange happens through this organ. We wanted to come up with a model system that represents a placenta that was cell free and didn’t involve using an animal. So, what we did was we first studied cells taken from a placenta and the lipid composition of these cells, and then we made lipid bilayers out of synthetic lipids that mimicked the composition of placental cells at different trimesters during the pregnancy. And then we looked at how different small molecules (some of them were actually antimicrobial therapeutics) interact with these synthetic lipid bilayer models.
We noted the differences between the different trimesters and compositions of the placental cells in terms of the lipid content and how these toxicants, small molecules and pharmaceutical agents interacted. It’s early stage work but that same technology could be adapted for the purpose of high throughput testing in a cell-free environment for a range of applications.
What you do in your lab has a real-world effect. How important is that?
We’re very real-world application driven. I think the science is great, and we do a lot of fundamental science in the lab too, but the purpose is to solve real-world problems. Right now, with the pandemic, the work we’re doing on antimicrobial drug delivery is very relevant. The data show that bacteria and fungal co-infections for patients that have Covid-19 are increasing greatly and that’s heavily problematic. The antimicrobial resistance issue is just going to be exacerbated because these patients can also receive antibiotics and antifungals at the same time.
Finding solutions to real-life problems at the Shukla Lab. Image courtesy of Brown University School of Engineering
How did you get to this point in your career?
The one big factor in where I ended up is my family. My family has always supported me tremendously and I’ve had a very positive role model of an academic and researcher in my father. That definitely got me early exposure, which exemplifies and solidifies the fact that early exposure is really important, which can come from your family, friends, teachers, and other role models.
When I started my undergraduate studies at Carnegie Mellon University, I thought I wanted to go into medicine at first, but then when I got there. I really enjoyed designing solutions that physicians would use. As an undergrad, I didn’t really know what I wanted to do in terms of the exact field of research; so, every summer I did a different research experience. In the first summer, I worked at the University of Rhode Island in a Mechanical Engineering lab. For the second summer, I worked at MIT in a materials science lab. And for my third summer, I worked in Columbia University in applied physics and mathematics. I also did research at Carnegie Mellon University with a faculty member in chemical engineering and just tried to get mentors and different experiences under my belt so I could get better informed in what I wanted to do. I then went to MIT to study chemical engineering for my graduate degrees.
Did any specific people help you along the way?
I worked with a faculty member at MIT, Paula Hammond, who’s now the department Head in Chemical Engineering at MIT. She was really an amazing influence for me. I definitely had strong female role models as an undergrad, but my graduate supervisor at MIT happened to be a strong black female scientist and that was hugely influential to me – to see that you can be a minority in STEM, really successful, and do it all. At the same time, she was very open about challenges for women in chemical engineering and not afraid to talk about it at all. She did a great job in promoting us and making sure we had the right mentoring during the five years of my PhD. So, I’m very grateful to her.
I did my postdoc at Rice University in the bioengineering department, and I worked with another really strong female mentor there. My postdoctoral advisor, Jennifer West – who is now the Dean of Engineering at the University of Virginia – was really amazing. I learnt a whole new set of things from her. In all of this, I can pinpoint that I’ve had many mentors. I would highly advise that regardless of what you are interested in doing in life, find those people who are out there to support you.
How did you end up at Brown?
I ended up at Brown in the School of Engineering as a tenure track assistant professor in the summer of 2013. Since then, all the time has gone into setting up my lab and advancing our science. It’s pretty much flown by. I’ve been extremely lucky. I’ve had amazing students and postdocs in my lab. They really produce everything that comes out of it. I’m just the spokesperson.
I love working with them. We have a very inclusive environment. We talk about a lot of diversity, equity, and inclusion-related concerns. I think that’s really important. We try to self-educate and educate each other on these topics. We have a welcoming environment and genuinely care that everyone in the lab feels respected. Because you can only do good science and good work if you work in a place where you are happy and respected and can be yourself.
What does a given working day look like?
It varies. A given day is chaotic due to work and having two small kids. My husband is also a professor at Brown so we both have similar demands on our time but a lot of my time goes into research and proposal writing. We need to raise funds to run a lab so we definitely spend a lot of time on that. Paper writing to get out work out is also super important.
My favourite things are meeting with my grad students and postdocs about research. I love meeting with them and talking with them about their data and generating new ideas together. This semester I am also teaching a class about advances in biomedical engineering over the past couple of years. Preparing those classes and making sure I am devoting time to them is important to me.
‘One thing I always tell students is don’t doubt yourself. Go ahead and try.’ Image courtesy of Brown University School of Engineering
What challenges have you had to overcome in your career?
I've been extremely lucky, but there has been the two-body situation. It’s essentially having a working spouse and trying to figure out how to make it work so that you both have the careers you want in the same location. That took me and my husband five years to figure out.
My husband was in Texas and I was in Rhode Island and I had two babies with me while doing this academic career on my own. That’s incredibly challenging, but it’s extremely common. In general, I think industry and academia need to work harder to make it easier for individuals to figure out this situation and smoothen the transition.
There are other little things that come up that are challenging. I do often feel that I have to prove myself to my older male colleagues at times when I shouldn't have to. If I get into an elevator with a male colleague who’s exactly the same age as me, a senior male colleague might ask that colleague about his research, and I might be asked about my kids. I often think it’s not intentional – and I try to give people the benefit of the doubt – but I think there’s a lot of education that still needs to be done.
>> Interested in the latest on antimicrobial drug discovery? Register to attend the 3rd SCI-RSC symposium on antimicrobial drug discovery on 15 and 16 November.
What’s the current state of play in your sector with respect to diversity, equality, and inclusion?
There's a lot to do but there’s a lot more awareness now. We’re far from where we need to be in terms of representation of all sorts of individuals in academia. Really, it’s ridiculously appalling if we look at numbers of black individuals, women in STEM academics, or the grant funding that goes to these individuals. But I have seen over the past two years or so that there’s just been more people talking about it. In biomedical engineering, a group of around 100 faculty or so academics around the US gets together periodically over Zoom to talk about these topics, and there’s more awareness and content in our scientific forums.
What’s the greatest challenge for people developing antimicrobial materials or in biomedical areas?
With therapeutics, it’s the FDA approval timeline. It’s years later by the time they’re used. A lot of the time people shy away from working in therapeutics because they know how hard it is going to be to commercialise something in that area.
On an academic level for me as an engineer, it’s critical to figure out what the important challenges and problems are. We’re very lucky at Brown that we have a great medical school so we can talk to clinicians, but cross-talk between disciplines is super important right now.
What advice would you give to young professionals in your area?
One thing I always tell students is don’t doubt yourself. Go ahead and try. You can’t win a game if you don’t play it. I constantly run into individuals who say: ‘I didn’t apply for that because I didn’t think I was qualified’. Basically, I just tell them to apply – you have nothing to lose.
What are you and your students working on that you’re most excited about at the moment?
I really love everything we are doing! I love the fact that we are designing materials that are smart, so they respond to the presence of microbes. I think that could be groundbreaking in terms of prolonging the lifetime of our existing antimicrobial drugs. We also have some really great work going on in treating biofilms, which are incredibly problematic in terms of infections. It’s very hard to answer. I’m proud of everything we do.
>> In recent months, we’ve spoken to inspiring women who work in science. Read more about the stories of materials scientist Rhys Archer and Jessica Jones, Applications Team Leader at Croda.
Our careers often take us in unforeseen directions. Dr Jessica Jones, Applications Team Leader at Croda, chatted to us about moving from research into management, the benefit of developing softer skills, and her unexpected mentor.
Tell me about your career to date.
I came through university in what is probably seen as the ‘traditional’ way. I did a Master’s degree in chemistry at the University of Liverpool, with a year working in industry, which I really enjoyed. And then after I finished my Master’s, I did a PhD in Inorganic Chemistry at the University of Nottingham. I always wanted to work in industry, but I really enjoyed research, so I decided to do the PhD as I thought the skills would be useful for either career path.
Jessica Jones in the lab
Were you tempted by a career in academia?
No, I never felt like I was the kind of person who had what it takes to succeed in academia. I never felt like I could ever come up with the nucleus of a new idea. I always felt like someone could give me the slimmest thread of a thought and I could turn it into something, but I could never have that thread myself. From my perspective, academia can be a lonely career and I enjoy and benefit from working in a team with other people.
So, after I finished my PhD, I joined Croda in 2013 as a Research Scientist in our synthesis division, in a synthetic chemistry R&D role. Over seven years, I progressed from Research Scientist to Lead Research Scientist and then Team Leader. During that time, I moved around a bit. I worked at different manufacturing sites, in different research areas and did lots of different projects across multiple sectors.
In February 2020, I was asked if I wanted to go on secondment, as a Team Leader, to one of our applications teams in Energy Technologies. Energy Technologies focuses on lubricants, oil and gas, and batteries. I really enjoyed the secondment and after it came to an end, I chose to take it on as a permanent position rather than return to my old role.
What does this role entail?
My role entails managing a team of application and lead application scientists who work on a range of projects, from designing new products to supporting customers with specific problems and working with universities on more theoretical, developmental ideas.
At the moment, we’re working on a lot of what we call EV (electric vehicle)-friendly fluids. When you move from traditional combustion engines to electric vehicles, there’s quite a change in the properties needed for the fluids within the engine. We make the speciality additives that go into the base oils that support functions such as reduced engine wear and improved fuel efficiency.
The EV market is very different to the traditional car market, which is dominated by big lubricant manufacturers. EVs are so new that Croda has been at conception discussions with world leading EV companies. The whole sector is very data driven and, coming from a research scientist background, that appeals to me very much. It’s very exciting to be at the cutting-edge of innovation with what we’re doing within electrification and renewable energy.
Which projects are you working on at the moment?
I’ve got two long-term new development projects that are both progressing to the final stages of manufacturing. These are products that I designed the chemistry for when working in the synthesis team. It can take four or five years to get a new project through the development process, and I’ve continued to manage them throughout their timeline, even though I have moved into different roles. They are both speciality additives for crude oil to reduce the temperature at which impurities develop, to allow the more difficult oil fractions to be brought out of the ground without it solidifying in pipes when they transport it.

What does a general working day involve?
There are eight people in our team, and I am responsible for managing six of them. There are two other senior technical specialists I work alongside. They have lots of experience in the industry and working with academia, and the three of us coordinate the projects across the team.
My role is to translate the pipeline and the strategy from our senior leaders into what we do in the lab every day. I have three projects that I'm running, which are new product launches. Alongside that, I coordinate the project pipeline and make sure everyone is able to manage their projects and progress them. I do a small amount of lab work, but I would say it makes up 5% of my time.
I always thought I would be a specialist when I joined Croda because of my PhD and lab experience. However, over the time I’ve worked here, I started to really enjoy working with other people; and I think I probably realised I had better skills at motivating other people, building up teams, and networking. So that became a lot more important, and I chose to move into the management side of things but still within a technical function.
Interpersonal skills are sometimes underrated in management. How do you approach this side of the job?
I think I am quite at ease around other people as I am very extroverted. I think that makes me different from a lot of people in my team. For example, my boss and I are the total opposite of each other, but it works really well because it means that we complement each other perfectly. He’s very strategic and he likes to take his time to make decisions. He likes to review all the data very methodically and is good at using detail to evaluate a project’s true value, whereas I’m much more about talking to people, bringing everyone together and acting quickly to get things done. But I think the balance of both works incredibly well for us as a team.
During lockdown we received a webinar on personal resilience, and the session was about your outward projection to other people. About 70% of how you are perceived by others is made up of how people see you and your ‘brand’. Your technical expertise and actual ability to do your job only makes up about 20% of how people view you and how successful you are. And I think as a scientist, you get a bit focused on delivering the project successfully, thinking that you need to be really amazing at delivering data, but people forget about the need to work on themselves to develop as well.
What part of your job motivates you most?
It’s a combination. The science we’re working on is very exciting, and I really enjoy getting all the projects together, making sure everything fits together and that everyone’s doing the right thing. But emotionally, it’s the team that gets me up in the morning – coming in, seeing what they do, how they have been. I’ve been really lucky over the past 12 months, being able to see some of my colleagues really develop. I’ve taken a lot of pride in realising the impact you can have on other people and allowing yourself to take credit for that.
>> What is life like as a materials scientist? Take a look at our thought-provoking conversation with Rhys Archer, founder of Women of Science.
Which mentors have helped you along the way?
There’s one person who stands out. I was asked to take on this extra role to become a European technical rep in one of our business areas. I’d never done anything like that before so the idea that I was going to be put out there, in front of customers, as the technical expert for the business was quite terrifying.
I was to work with the European Sales Manager of the business, and we ended up traveling a lot together. He was the opposite to me. He’s very experienced but had a reputation as a bit of a loud, burly Yorkshireman and I wasn’t sure how we would fit together, but we got on like an absolute house on fire. He was so helpful to me, not just in giving feedback on what I was doing in the role, but general conversations about career and life outside of work and personal support. Having that kind of professional relationship develop has made a massive difference. Just meeting someone like that and having a person to go to when I needed help, someone who I really trust to have my best interests at heart. It was very beneficial for the number of years that we worked together. Since then, we have moved on to different roles, but we still stay in touch, and it has taught me the value in reaching out to different people to help me to develop.
Jessica with the first product she developed at Croda.
In terms of equality and diversity, do you think enough is being done in your sector?
I think there is always more that can be done but I’ve never felt my gender has hindered me in my career and I’ve always felt very supported at Croda. Sometimes people are in a rush to see change immediately, especially when the senior management at Croda and many other STEM organisations is still made up of a majority of white males.
I like to think that the support myself and others have been given will mean that, as we progress, there will be more representation in senior positions. I would always want to achieve something on merit rather than to tick a box for equality. If that means it will take time for the generation I am in now to get to those positions, then I can wait. Importantly, I genuinely think everything that’s being put in place at Croda, and more broadly across the STEM sector, will pave the way for more diverse representation in senior roles in the future.
Do you have any advice you’d give to someone starting out?
Having a mentor is very important. I never thought I needed one until accidently developing that relationship. Since moving into different roles, I’ve set out to deliberately engage with people for that purpose. I would encourage people to seek out those who are different from themselves and engage with them.
I also think it’s important not to be afraid to ask for things you want. If you want to get a promotion or seek out further development, it’s often tempting to ask permission. If you can demonstrate to people that you are ready, it is more effective.
Generally, I think people, especially women, really underestimate the value of self-promotion as they worry it can be perceived as arrogance. A lot of people think that if you simply do a good job, then you’ll be recognised for that. That would be amazing if it were true, but people will judge you on how you’re perceived and how you present yourself, as well as what you do.
I think you need to put yourself out there. Whether it’s getting involved in something outside of your day job or taking the lead in a particular task, it’s a great way to get recognised. Sometimes it won’t work out and it can be hard to take the criticism when that happens, but you always learn from the outcome. I always prefer to have given something a go, even if I fail, than never to try.
Finally, I think people should always be themselves because everyone has unique skills to offer. I don’t think people would look at me and think that I look like the manager of a technical team, but I’m comfortable with my own style and that makes other people comfortable with it too.
>> We’re always interested in hearing about different people’s diverse career paths into chemistry. If you’d like to share yours, get in touch with us at: eoin.redahan@soci.org
A group of inspiring young scientists took centre stage at COP26 on 4 November to show how the next generation of chemists is finding tangible climate change solutions.
In a day dominated by what countries pledged to stop doing at COP26, such as pursuing coal power and financing fossil fuel projects overseas, it was refreshing to learn about low-carbon technologies and the young people driving their development. At the Next Gen forum, we heard from an array of young chemists, all associated with SCI, who are at the sharp edge of this change.
We heard from Brett Parkinson, Senior Engineer of Low Carbon Fuels and Energy Technologist at C-Zero, who is working on commercialising a way to decarbonise natural gas. The California-based company’s technology converts the natural gas into hydrogen and solid carbon to provide a clean energy source while sequestering the carbon; and the aim is to have this process up and running next year.
Natasha Boulding is building towards Net Zero a different way – with a greener concrete. The CEO and Co-founder of Sphera has developed a lightweight carbon negative additive using waste plastics that aren’t currently being recycled. She says the company’s blocks are the same strength and price as existing concrete blocks, but with 30% more thermal insulation. There is also the added benefit of reusing waste materials that would otherwise have gone to landfill or been incinerated.
Another solution discussed by Dominic Smith, Process Development Engineer at GSK, reduces energy consumption through green chemistry. He is trying to find greener ways to make medicines using enzymes. These enzymes, which can be found in plants and soil, replace chemical synthesis steps to cut energy consumption during processing and reduce hazardous waste.
Panel (left to right): Dominic Smith, Natasha Boulding, Clare Rodseth, Jake Coole, Nikita Patel, and Oliver Ring (Brett Parkinson spoke via video link).
It was apparent from the discussion that many solutions will be needed for us to reach our climate change targets. On the one hand, Jake Coole, Senior Chemist in Johnson Matthey’s Fuel Cells team, is working on membrane electrode assembly for hydrogen fuel cells to help us transition to hydrogen-powered buses and trucks.
At the same time, Clare Rodseth, an Environmental Sustainability Scientist at Unilever, has been using lifecycle assessments to reduce the environmental impact of some of the 400 Unilever brands people use all over the world every day. For example, this work has helped the company move away from petrochemical ingredients in its home care products. ‘Even small changes,’ she said, ‘have the potential to bring about large-scale change.’
Incremental change
However, for each of the technologies discussed, barriers remain. For Coole and co., having a readily available supply of hydrogen and charging infrastructure will be key. And for Dominic Smith and his colleagues, the use of enzymes in green chemistry is still in its infancy; and getting enzymes that are fast enough, stable enough, and produce the right yield is difficult. Nevertheless, he noted that manufacturers are now using enzymes to produce the drug amoxicillin, reducing the carbon footprint by about 25%
And some things will take time to change. Natasha Boulding noted that concrete is the second most used material in the world after drinking water, and we simply can’t create many green technologies, such as wind turbines, without concrete foundations.
She said the construction industry is quite traditional but also pointed to perceptible change, with the green concrete market growing and companies becoming increasingly aware of their carbon footprints.
Collaboration was seen as crucial in producing climate change solutions.
The reality is that global action on climate change is recent. As Brett Parkinson said: ‘the main reason we’re talking about it now is that there’s a driver to do it. Until the last decade, the world hadn’t cared about CO2 emissions. They just talked about caring about it.’
How pivotal is science in all of this?
So, what could be done to make climate action more effective? For Parkinson, effective policy is key. He argued that if the market isn’t led by policies that encourage low-carbon innovations, then it won’t work as needed. ‘It all starts with effective decarbonisation policy,’ he said. ‘Legacy industries are very resistant to change. If you don’t have strong and consistent policies… then they’re not going to adapt.’
Another key to our low-carbon evolution is collaboration, and the SCI provides a confluence point for those in industry and academia to work together to produce innovative, low-carbon products. As Clare Rodseth said: ‘Collaboration is really important – linking up people who can actually come together and address these problems.’
As the discussion came to a close, you had the impression that the debate could have gone on for much longer. ‘Hopefully, we’ve demonstrated that there is action, and it’s being driven by young people like our panellists today,’ summarised Oliver Ring, the event’s co-Chair, before asking for the result of the audience poll.
The question: How many of those watching believed that science is pivotal in providing climate change solutions?
The answer: Just the 100%.
>> Thank you to Johnson Matthey for sponsoring the event, to the speakers for sharing their time and expertise, and to co-chairs Nikita Patel and Oliver Ring for doing such an excellent job.
This Thursday at COP26, an inspiring panel of young scientists will discuss innovations that will help us mitigate climate change. So, what can we expect?
Millions of young people are frustrated by climate change inaction. Indeed, according to a University of Bath study, 60% of the next generation feel overwhelmed by climate anxiety. Often, the proposed solutions seem vague and intangible – well-intentioned ideas that drift away when the political winds shift.
And yet, when you see the ingenuity of young scientists, business people, and activists, it’s hard not to be excited. Undoubtedly, politics and our legal system will play a huge role in the drive to reach Net Zero, but arguably science will play the biggest role in transforming the way we live. Just think of the falling cost of generating solar power, improvements in battery chemistry for electric vehicles, the development of sustainable construction materials, and the rapid rollout of Covid-19 vaccines.
Tangible solutions
This Thursday at COP26, SCI will host the Next Gen youth forum event where the panellists discuss the climate change solutions they are working on right now and how they are being applied by industry. In the Countdown to Planet Zero roundtable, these scientists – drawn from within SCI’s innovation community – will explain their work to a global audience and the impact it will have on climate change.
They will discuss innovation in three key areas: topics of fuels of the future, turning waste into gold, and engineering nature.
The next generation has mobilised and is creating solutions to help avoid climate change disaster.
The panel will be chaired by two very capable young scientists. Oliver Ring is Senior Scientist at AstraZeneca’s large-scale synthesis team and Chair of SCI’s Young Chemists’ Panel, and passionate climate advocate Nikita Patel is a PhD student at Queen Mary University of London’s Centre of Translational Medicine and Therapeutics and STEM Ambassador for schools.
The other panel members include Clare Rodseth, of Unilever’s Environmental Sustainability Science team, who brings lifecycle analysis to product innovation to make products more sustainable.
Jake Coole, Senior Chemist in Johnson Matthey’s Fuel Cells team, is involved in the scale-up of new processes and next generation manufacturing, and Dominic Smith, Process Development Engineer at GSK, who is interested in engineering biology to create sustainable manufacturing processes.
Also present will be Dr Brett Parkinson, Senior Engineer of Low Carbon Fuels and Energy Technologist at C-Zero – a California-based startup that works on the decarbonisation of natural gas. In 2019, Brett was awarded an SCI scholarship for his research.
The lineup also includes Dr Natasha Boulding, CEO and Co-founder of Sphera Limited, a speciality materials company that has created carbon negative concrete blocks made from aggregate including waste plastic. According to Natasha, whose company also won SCI’s Bright SCIdea challenge in 2019: “In terms of combating climate change, interdisciplinary collaboration is the key. No one discipline has the answer to solve our biggest challenges – but together diverse minds can.’
>> Would you like to take part in BrightSCIdea and be in with the chance of winning £5,000? Be part of it.
Watch the event online
SCI is proud to be associated with these enterprising young scientists and the imaginative solutions they are developing to mitigate the effects of climate change.
‘As a global innovation hub, SCI wants to show how the next generation of scientists is actively developing solutions,’ said Sharon Todd, SCI CEO.
Sharon Todd, SCI CEO
‘Our COP26 youth forum debate will profile the work of young scientists and entrepreneurs addressing climate change in their work. This next generation of innovators has the power to change our world’s tomorrow.’
If you’d like to see the climate change solutions of tomorrow, register to watch the virtual event here.
Continuing our series on Black pioneering scientists and inventors, we profile Garrett Augustus Morgan. His observations led him to upgrade the sewing machine, invent and upgrade life saving devices and develop personal care products for Black people, while championing civil rights and fighting for his own recognition.
Garrett Augustus Morgan | Image credit: Public domain image courtesy of: https://www.dvidshub.net/image/1165661
Garrett Augustus Morgan was born in 1877, in Kentucky, US. Like many Black students he left school at a young age to find work. However, while working as a handyman in Cincinnati, he was able to hire a tutor and continue his studies.
During 1895, Morgan moved to Cleveland, Ohio, and it is said that Morgan’s interest in how things worked was sparked while repairing sewing machines for a clothing manufacturer. It was during this time that Morgan’s first inventions were developed: a belt fastener for sewing machines and the attachment used for creating zigzag stitching. By 1905, Morgan had opened a sewing machine shop and then a shop making clothes, ultimately providing employment for more than 30 people.
It was also during this time that Morgan became involved in the establishment of the Cleveland Association of Coloured Men. In addition to his interest in ‘gadgets’, Morgan also patented hair care products for Black people.
The life-saving Safety Hood
Morgan is credited with several inventions that have been responsible for saving many lives. In 1912 he filed a patent for the Safety Hood, which was developed after he had seen fire fighters struggling from the smoke encountered while tackling blazes. On the back of his invention, Morgan was able to establish the National Safety Device Company, in 1914, to market the product. While Morgan was able to sell his safety device across the US, it is said that on some occasions he hired a White actor to take credit for the device, rather than revealing himself as the inventor.
Morgan’s Safety Hood was soon in use in various settings including hospitals and ammonia factories. Indeed, the Safety Hood was used to save many lives and by the start of World War I, the breathing device had been refined to carry its own air supply. The Safety Hood was awarded a gold medal by the International Association of Fire Chiefs.
>> Read more about trailblazing Black scientists here.
Morgan’s device reached national prominence when it was used in the rescue of survivors and victims of a tunnel explosion under Lake Erie in 1916. The accounts tell of Morgan being woken early in the morning of 24 July 1916, after two rescuers lost their lives following the explosion.
Morgan is said to have arrived on the scene in his pyjamas, with his brother and a number of Safety Hoods. To allay the fears of the sceptics about his Safety Hood, Morgan went into the tunnel and retrieved two victims. Others joined and several people were rescued. Morgan is reported to have made four trips, but this heroism affected his health for years after as a result of the fumes he encountered.
Sadly, Morgan’s bravery and the impact of his Safety Hood were not initially recognised by the local press or city officials. It was some time later that Morgan’s role was acknowledged; and in 1917 a group of citizens presented him with the gold medal.
Garrett A. Morgan rescues a man at the 1917 Lake Erie Crib Disaster | Creative Commons CC BY-SA 3.0 Image in the Public Domain
While orders for Morgan’s device increased following the incident, it is said that when his picture appeared in the national press, crediting him as the Safety Hood inventor, officials in a number of southern cities cancelled their orders. Morgan is quoted as saying; ‘I had but a little schooling, but I am a graduate from the school of hard knocks and cruel treatment. I have personally saved nine lives.’
Safety seemed to be an important area for Morgan, as he became alarmed about the number of accidents that were occurring as cars became more prevalent in America. Along with the cars, bicycles, animal-drawn carts and people were sharing ever more crowded roads.
After witnessing an accident at a junction, Morgan filed a patent for a traffic light device which incorporated a third warning position. The idea for the ‘all hold’ position or what is now known as the amber light was patented in 1923. Morgan sold the idea to General Electric for $40,000 the same year. It should be noted, however, that a three signal system had been invented in 1920.
Morgan is credited with establishing a newspaper, building a country club open to Black people, and running for a seat on the Cleveland City Council, among many notable achievements. Morgan died in July 1963. He has been recognised in Cleveland Ohio, with the Garrett A. Morgan Cleveland School of Science, and the Garrett A. Morgan Water Treatment Plant being named in his honour. In addition, a number of elementary schools and streets carry his name.

Sharon Todd, SCI CEO
It’s always great to meet new – and old – contacts at events. For so many months, crossing borders wasn’t possible, physically at least, due to the Covid-19 pandemic. Thankfully, the Chemical Innovation Conference (CIEX) provided a welcome change.
On 6 and 7 October, we came together in Frankfurt to discuss the challenges and opportunities in our sector. It was an honour for me to give the opening address – in the same year as SCI’s 140th anniversary.
Indeed, this year’s event included a well-paced mixture of talks and panel events that addressed post-pandemic difficulties, the challenges of climate change, the need to innovate and much more.
The chemical using industries face an array of challenges besides the practical fallout from the Covid-19 pandemic. Brexit, new regulations, supply chain issues, climate change, sustainability, and geo-political unrest pose significant problems
As an innovation hub, SCI connects industry, academia, patent lawyers, consultants, entrepreneurs and government, and other organisations. And I like to think of CIEX as an innovation hub too.
We have no choice but to innovate, but we must do so in a collaborative, sustainable way. The climate change emergency, for example, means society is looking to chemistry to help find long-term innovative solutions. That’s what made CIEX such an apt time for those in the industry to come together and navigate these challenges.
Innovating beyond barriers
The theme of this year’s event was ‘Game-Changing through Collaboration’. But I also thought of it as Crossing Borders – not just physical borders, but getting through the barriers that block innovation. These barriers hold back the translation of scientific solutions from the laboratory into business and, ultimately, into society.
Our sector is in the spotlight as never before and we can shape a better future. The debates at this year’s CIEX, and the exchange of ideas that took place, will help move us all forward. And what an exchange of ideas it proved to be.
We heard from an amazing line-up of speakers, addressing some of the industry’s most salient issues. BASF’s Christian Beil spoke about how best to leverage lean experimentation and rapid prototyping to improve customer centricity in product design, while Iris AI’s Anita Schjoll Brede described how we can reimagine the R&D work environment.
Ineos Styrolution plans to recycle polystyrene using thermal decomposition or by washing and remelting waste.
Furthermore, Johnson & Johnson’s Luis Allo spoke about the rise of consumer awareness as a driver for innovation. He provided interesting insights on accessing information on real customer trends and needs. Dupont’s Fred Godbille also described several tried and tested methods to assess the voice of the customer.
Elsewhere, Croda’s Nick Challoner assessed how we can unlock innovation through collaboration and partnerships. He also provided an overview of how Croda interacts with universities. On a more technical note, Roman Honeker of Ineos Styrolution outlined the company’s plans to recycle polystyrene using thermal decomposition or by washing and remelting waste.
The discussion on ‘how SMEs interact with corporates’ provided another of the event highlights, with contributions from Clariant, BASF, Chemstars, and SCI’s David Bott. Delegates discussed how SMEs sometimes oversell the potential of their products (without necessarily having much real-world experience) and the allegedly slow-moving, risk-averse nature of some corporates.
Cross-border innovation
Throughout the event, attendees examined what we can do better, how this can be achieved, and the resources needed to make this happen. After all, we must be nimble and flexible in these times of political and social uncertainty.
We can cross borders together – physically and virtually – via close collaboration. And we can cross the borders of what’s possible innovation-wise, removing barriers and journeying into new territory for us all.
To celebrate Black History Month, we take a look back at some of the great Black scientists and innovators. From laser eye surgery to the gas mask, here are some of the seminal contributions made by these ingenious inventors.
[1] Lewis Howard Latimer – Image credit: Unknown author Unknown author, Public domain, via Wikimedia Commons
[2] Leonidas Berry - Image credit: Adundi, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
[3] Betty Harris – Image credit: https://www.blackpast.org/african-american-history/harris-betty-wright-1940/ - Fair use image
[4] Patricia Bath - Image credit: National Library of Medicine, Public domain, via Wikimedia Commons
[5] Philip Emeagwali - Image credit: SakaMese, CC BY-SA 4.0, https://creativecommons.org/licenses/by-sa/4.0, via Wikimedia Commons
1880 – Johnson Powell
Have you ever used eye protectors to protect yourself against the glare of intense light? For those working in extreme environments such as fires and furnaces, Johnson Powell’s eye protectors will have been a sight for sore eyes.

1881 – James Wormley
James Wormley invented a life-saving apparatus for boats. His contraption included a string of floats that extended from a ship’s side via a sliding rod with projecting arms. The famous hotelier was also said to be at President Abraham Lincoln’s bedside when he died.
Image Credit: Unknown author, Public domain, via Wikimedia Commons
1882 – Lewis Howard Latimer
Lewis Howard Latimer is probably best known for inventing a durable carbon filament that was key to the success of the electric light bulb. Latimer also invented an evaporative air conditioner and even drafted the drawings to secure the patent for Alexander Graham Bell’s little known invention… the telephone.
>> Click here for more on Lewis Howard Latimer’s extraordinary contribution to science.

1912 – Garrett Morgan
Imagine using your own invention to save people’s lives? That’s exactly what Garrett Morgan did when he donned his patented smoke hood to rescue trapped men from a smoke-filled tunnel beneath Lake Erie. Morgan’s device later evolved into a gas mask, and he also invented a three-position traffic signal, hair straightening cream, and a self-extinguishing cigarette for good measure.
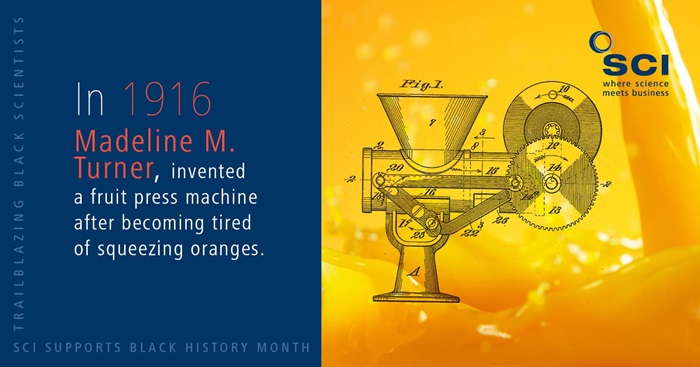
1916 – Madeline M. Turner
Madeline M. Turner’s ingenious invention was the fruit of her own frustration. Turner grew tired of squeezing oranges for her glass of juice, so she created the fruit press machine to solve the problem.
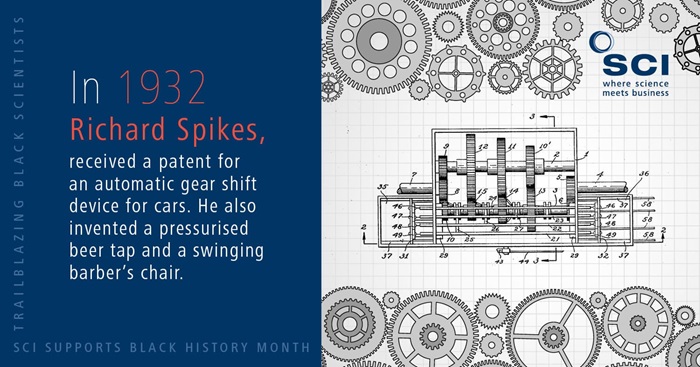
1932 – Richard Spikes
It’s safe to say Richard Spikes was a polymath. The American inventor created an automatic gear shift device for cars, a pressurised beer tap, and a horizontally swinging barber’s chair – all while working as a teacher and barber and being a capable pianist and violinist.

Image Credit: Adundi, CC BY-SA 4.0, via Wikimedia Commons
1966 – Leonidas Berry
This doctor and civil rights advocate invented the Eder-Berry gastroscopy endoscope in 1955, which helped doctors to biopsy the inside of the stomach without surgery. According to the US National Library of Medicine, ‘the Eder-Berry biopsy attachment made the gastroscope the first direct-vision suction instrument used for taking tissue samples during gastroscopic examination’.

Image Credit: https://www.blackpast.org/african-american-history/harris-betty-wright-1940/ - fair use image
1984 – Betty Harris
Perhaps the most explosive discovery of all belongs to Betty Harris. Harris’ spot test for detecting 1,3,5-triamino-2,4,6-trinitrobenzene in the field is used by US Homeland Security today to check for nitroaromatic explosives. In her spare time, Harris has even found the time to work with the Girl Scouts to develop a badge based on Chemistry.
>> SCI is proud to support #BlackinChem. Take a look at some of our recent work.

Image Credit: National Library of Medicine, Public domain, via Wikimedia Commons
1988 – Patricia Bath
Patricia Bath has helped return the gift of sight to thousands of people. The US ophthalmologist invented a quick and painless device that dissolves cataracts with a laser and cleans the eye, enabling the simple insertion of a new lens. Her laserphaco probe is still in use today.

Image Credit: Philip Emeagwali - SakaMese, CC BY-SA 4.0, via Wikimedia Commons
1989 – Philip Emeagwali
Nigerian computer scientist Philip Emeagwali won the prestigious1989 Gordon Bell Prize in Price Performance for a high-performance computer application that used computational fluid dynamics in oil-reservoir modelling. In the same year, Emeagwali also claimed to perform the world’s fastest computation – 3.1 billion calculations per second – using just the power of the internet.
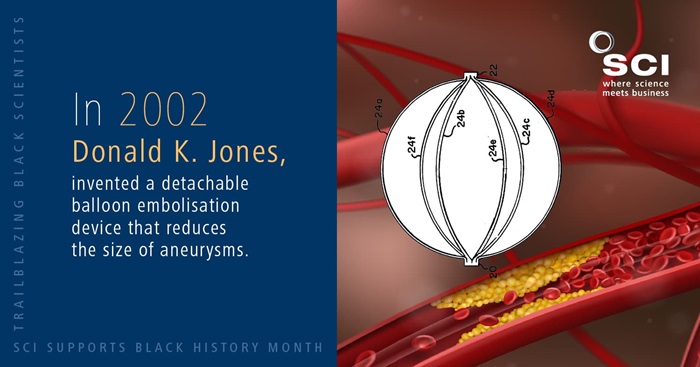
2002 – Donald K. Jones
Donald K Jones made a notable contribution to medicine with his invention of a detachable balloon embolisation device that reduces the size of aneurysms (bulges in blood vessels). The endovascular occlusion device is implanted into the body, whereupon its clever balloon system and adhesive materials reduce the size of aneurysms.
>> Which barriers still block the way for Black chemists? Read Claudio Lourenco’s story.
We need to create more diverse paths into research and scientific innovation. Professor Dame Ottoline Leyser, Chief Executive of UK Research and Innovation, explains how industry clusters and a change of mindset could help.
What do you picture when someone mentions a chemist? Maybe you see someone like you working in a lab or office with your colleagues.
But what do people at the bus stop think? What would a secondary school student say? Do they see someone like them – or do they imagine an Einstein-like figure hidden away in a dark room with crazed hair and test tubes?
One of the most interesting messages from Professor Dame Ottoline Leyser’s Fuelling the Future: science, society and the research and innovation system talk on 29 September was the need to make sure science and technology are seen as viable careers for people throughout society.
Prof Dame Ottoline Leyser
You don’t need to be a genius to work in research and innovation. You don’t necessarily need to be a specialist, and you certainly don’t need to be hunched over a microscope with a jumble of figures and formulae on a board behind you. An array of different people, technical and non-technical, are needed to make the sector thrive.
The narrow pathway of talent
Part of Dame Ottoline’s job as Chief Executive of UK Research and Innovation (UKRI) is to improve access to these sectors and to make sure that great ideas aren’t lost due to daunting entry barriers.
‘It’s a huge challenge,’ she said. ‘A large part of the challenge is the narrow concept that we all have of what a researcher and innovator look like.’
Leyser spoke about the need to create diverse routes through the system rather than squeezing everyone through the same narrow path. ‘The assessment criteria we use for individuals have become narrower and narrower,’ she added. ‘Some of it, ironically, is to make the system fairer, but objectivity in creativity is a total pipe dream. You end up crushing creativity by narrowing the criteria.’
She noted that those with mixed careers – interwoven with varied experiences – are to be welcomed. ‘That’s nothing to do with compromising excellence,’ she said. ‘Real excellence comes in multiple forms.’
>> Would you like to attend more talks like this one? Check out our Events page.
Challenging times
However, Leyser also spoke of the need to level up the UK from a productivity perspective. One way to do this is through smart specialisation and industry clusters. She mentioned Lincoln as an area where this approach worked well. Lincoln is home to extensive agriculture and the multinational technology corporation Siemens. As such, it made sense to help make it a centre for agricultural robotics.
UKRI is investing heavily in research and innovation into Net Zero energy solutions.
As the largest public funder of research and innovation in the UK, UKRI has a major role to play in funding such industry clusters and intelligent innovation. It has funded more than 54,000 researchers and innovators, and UKRI grants have generated almost 900 spinouts since 2004.
These include Oxford Nanopore, a biotech company whose DNA sequencing technology is now valued at £2.5bn. It has also cast an eye on the future, including delivering more than £1bn in R&D relevant to Artificial Intelligence and in excess of £1bn towards Net Zero energy solutions.
Leyser noted that the UKRI’s goal is to embed research and innovation more broadly across society – for it to be ‘by the people and for the people, rather than the exclusive domain of the privileged few’.
It is a grand challenge, but such sentiments are certainly encouraging.
Continuing our series on Black scientists, Dr George Okafo tells us about his journey from curious child, encouraged by family and mentors, to Global Director of Healthcare Data and Analytics with a leading pharmaceutical company.

What is your current position?
I am Global Director, Healthcare Data and Analytics Unit at Boehringer Ingelheim, and have been in this role for the past 10 months.
Right: Dr George Okafo
Please give us a brief outline of your role.
To build an expert team of data stewards, data scientists and statistical geneticists tasked with accessing and ingesting population-scale healthcare biobanks and then deriving target, biomarker and disease insights from this data to transform clinical development and personalise the development of new medicines.
What was it that led you to study chemistry/science and ultimately develop a career in this field? Was this your first choice?
My interest in science stems from my parents. My father was a medical doctor, and my mother was a senior midwife. As a child, I was always very curious and wanted to know why and how things worked. This curiosity has stayed with me all my life and throughout my career at GlaxoSmithKline and now at Boehringer Ingelheim. In my current role, I am still asking the same types of questions from Big Data and these answers could have a profound impact in the development of new medicines.
Was there any one person or group of people who you felt had a specific impact on your decision to pursue the career you are in?
Yes, my father and mother, who supported, encouraged and gave me the confidence to be curious, to keep trying and to never give up.
Dr Okafo held senior director-level roles in drug discovery and development while at at GSK.
Could you outline the route that you took to get to where you are now, and how you were supported?
My career journey started at Dulwich College (London) where I studied Chemistry, Biology, Maths and Physics at A Level. This took me to Imperial College of Science, Technology and Medicine (London), where I completed my Joint BSc in Chemistry and Biochemistry and my PhD in Cancer Chemistry.
I then spent a year at the University of Toronto in Canada as a Postdoctoral Fellow, before embarking on my career in the Pharmaceutical Industry, starting at GlaxoSmithKline (GSK). I spent 30 years at GSK, where I held many senior director-level roles in drug discovery and development. During my time, I made it my mission to learn as much about the R&D process and used this knowledge to understand how innovation can impact and transform drug research.
I have been very fortunate in my career to be surrounded by many brilliant and inspirational people who had the patience to share their knowledge with me and answer my many questions.
>> Curious to read more about some of the great Black scientists from the past? Here’s our blog on Lewis Howard Latimer.
Considering your own career route, what message do you have for Black people who would like to follow in your footsteps?
Surround yourself with brilliant people who can inspire you. Look for people who you respect and can coach and mentor you. Don’t be afraid to fail. Work hard and keep trying.
What do you think are the specific barriers that might be preventing young Black people from pursuing chemistry/science?
No, I do not see colour as a barrier nor a hindrance to pursuing a career in science. I think it is important to look for role models from the same background to help inspire you, to answer your questions and to encourage you.
What steps do you think can be taken by academia and businesses to increase the number of Black people studying and pursuing chemistry/science as a career?
Have more role models from different backgrounds. This sends a very powerful message to young people studying science reinforcing the message… I can do that!
Could you share one experience which has helped to define your career path?
Not so much an experience, but a mindset – staying curious, inquisitive, always willing to learn something new, having courage that failure is not the end, but an opportunity to learn.
Marking Black History Month and following on from the #BlackInChem initiative, SCI is continuing its look back at some of the unsung Black scientists who pioneered, and made important contributions, to the advancement of science.
Today we profile Lewis Howard Latimer, much admired by his contemporaries; Alexander Graham Bell and Thomas Edison, but sadly a name, and story, that is not as well known.
Lewis Howard Latimer | Image Credit: By Unknown author - http://www.lrc.rpi.edu/resources/news/pressReleases/img/Lewis.jpg, Public Domain, https://commons.wikimedia.org/w/index.php?curid=2032528
Lewis Howard Latimer, the youngest of four children, was born in Chelsea, Massachusetts, on 4 September 1848. His father, George Latimer, a slave who had escaped, became something of a cause celebre when his owner recaptured him. However, abolitionists took his case to the Supreme Court and his freedom was secured.
Lewis proved to be an excellent student, with a particular flair for drawing, as well as writing poetry and stories, but lack of finance and restricted access to education meant that by 15 years of age, Lewis had joined the US Navy. The history books indicate that he was honourably discharged in 1865; when the Civil War ended.
Soon after, Latimer found work as an office boy with the patent firm Crosby, Halstead and Gould. It is here that combining his talent for drawing, and developing the skills of a draughtsman he was eventually promoted to the position of head draftsman. The history books record that Latimer’s first patent, in 1874 with colleague Charles Brown, was an improved toilet system for railroad cars.
Lewis Latimer was instrumental in helping Alexander Graham Bell file his patent for the telephone ahead of his competitors.
Latimer had many inventions, but it could be argued that his drawings for Alexander Graham Bell’s telephone, helped seal his place in science history. The story goes that Bell was in a race against time, as rivals were also looking to gain patent rights for a similar device. Bell hired Latimer who used his expertise in drawing and submitting patent applications to help Bell file his patent just hours, it said, before his rival in 1876.
By 1880 Latimer had taken up the post of mechanical draughtsman for the inventor Hiram Maxim, who was also the founder of the US Electric Lighting Company. Now focused on incandescent lighting, Latimer along with Joseph Nichols, invented a light bulb which used a carbon filament, an improvement on Thomas Edison’s paper filament. The invention, patented in 1881, was sold to the US Electric Lighting Company in the same year.
Latimer invented a process for making carbon filaments for light bulbs | Editorial credit: Claudio Zaccherini / Shutterstock.com
1A booklet by the Thomas Alva Edison Foundation noted; ‘Latimer invented and patented a process for making carbon filaments for light bulbs. He taught the process to company workers, and soon it was being used in factory production. Latimer also assisted in installing Maxim lighting systems in New York City, Philadelphia, Montreal and London. During the installation of lighting in Montreal, where a lot of people spoke only French, Latimer learned the language in order to competently instruct the workers. In London he set up the first factory for the Maxim-Weston Electric Light Company. That required him to teach the workmen all the processes for making Maxim lamps, including glass blowing. In just nine months Latimer had the factory in full production.’
In 1882 Latimer left Hiram Maxim and in 1884 joined the Edison Electric Light Company, where he was given the title draughtsman-engineer. In 1890 he joined the Edison Legal Department, and in 1893 testified in a case where the company said that its incandescent lamp patents had been infringed. In 1896 the Board of Patent Control of GE and Westinghouse was formed and Latimer became its Chief Draughtsman. He continued in that role until 1911 when he joined the consulting firm Edwin W Hammer.
On 24 January 1918, Latimer was named one of the 28 charter members – and the only African-American member – of the Edison Pioneers, ‘a distinguished group of people who worked to keep the ideals of Thomas Edison alive.’ The Edison Pioneers helped create the US’ electric power industry.
Latimer received patents for several inventions, including the safety elevator. He also had a passion for social justice. In a letter written in 1895 in support of the National Conference of Coloured Men, Latimer wrote: ‘I have faith to believe that the nation will respond to our plea for equality before the law, security under the law, and an opportunity, by and through maintenance of the law, to enjoy with our fellow citizens of all races and complexions the blessings guaranteed us under the constitution.’
Latimer died on 11 December 1928. Edison Pioneers historian and long time private secretary of Thomas Edison, William H. Meadowcroft wrote1 ‘Lewis Howard Latimer was of the coloured race, the only one in our organisation, and was one of those to respond to the initial call that led to the formation of the Edison Pioneers, January 24 1918. Broadmindedness, versatility in the accomplishment of things intellectual and cultural, a linguist, a devoted husband and father, all were characteristics of him, and his genial presence will be missed from our gatherings…We hardly mourn his inevitable going so much as we rejoice in pleasant memory at having being associated with him in a great work for all peoples under a great man.’
1For more information on Latimer’s life, work and legacy, see the Edison Electric Institute resource: Thomas Alva Edison Associate: Lewis Howard Latimer: A Black Inventor.
The War on Plastic is a grand title. To most of us, it doesn’t seem like much of a war at all – more like a series of skirmishes. Nevertheless, if you look closely, you’ll see that a lot of companies are tackling the issue.
GSK Consumer Healthcare (GSKCH) is one such organisation. The healthcare brand that gave us Sensodyne and Advil has launched a carbon neutral toothbrush to reduce our reliance on fossil fuels (which create virgin plastic).
The composition of its Dr. Best tooth scrubber is interesting. The handle comprises a mixture of a cellulose derived from pine, spruce, and birch trees and tall oil, which comes from the wood pulping industry. The bristles are made from castor oil and the plastic-free packaging includes a cellulose window.
According to GSKCH, Dr. Best is Germany’s favourite toothbrush brand and there are plans to apply the technology to toothbrushes across its portfolio, including its Sensodyne brand. At the moment, GSK needs to apply carbon offsetting initiatives to make the toothbrush carbon neutral, but it says it is working on future solutions that do not require this approach.
Net zero shopping
GSK isn’t the only company that is actively reducing the use of plastics and minimising waste. Supermarket chain Morrisons has made aggressive moves in recent years to cut waste, and has just launched six ‘net zero waste’ stores in Edinburgh that will operate with zero waste by 2025.
Customers at these stores will be able to bring back hard-to-recycle plastics such as food wrappers, foils, yoghurt tubs, mixed material crisp tubes, coffee tubs, batteries, and plant pots. At the same time, all store waste will be collected by a range of specialist waste partners for recycling within the UK, and unsold food will be offered to customers at a cheaper price on the Too Good to Go app.
Morrisons’ proactive approach will help find a new life for hard-to-recycle packaging.
‘We’re not going to reach our ambitious targets through incremental improvements alone,’ said Jamie Winter, Sustainability Procurement Director at Morrisons. ‘Sometimes you need to take giant steps and we believe that waste is one of those areas. We believe that we can, at a stroke, enable these trial stores to move from recycling around 27% of their general waste to over 84% and with a clear line of sight to 100%.
‘We all need to see waste as a resource to be repurposed and reused. The technology, creativity and will exists – it’s a question of harnessing the right process for the right type of waste and executing it well.’
If this approach is successful, Morrisons plans to roll out the zero waste store format in all of its 498 stores across the UK next year.
>> Interested in reading more about sustainability and the environment? Check out our blog archive.
Stamping out single-use plastics
The government has also issued its latest battle cry in the war on plastics. Having defeated plastic straws, stirrers and cotton buds, it has turned its attention to other single-use plastics.
Single-use plastic plates, cutlery and polystyrene cups are among the items that could be banned in England following public consultation.
The humble cotton bud has now been retired from active service.
Somewhat surprisingly, it estimates that each person in England uses 18 single-use plastic plates and 37 single-use plastic items of cutlery each year; so, it has begun moves to cut out this waste stream.
Environment Secretary George Eustice said: “We have made progress to turn the tide on plastic, banning the supply of plastic straws, stirrers and cotton buds, while our carrier bag charge has cut sales by 95% in the main supermarkets. Now we are looking to go a step further as we build back greener.”
All in all, it’s encouraging to see that companies and the government are brushing up on their sustainable practices.
>> Curious to find out what the future looks like for lab-processed food and meat alternatives? Read what the experts say here.
Life is busy for Rhys Archer. Outside of her work as EPSRC Doctoral Prize Fellow in Biomedical Materials at the University of Manchester, she founded Women of Science to share stories about real women working in science. She has championed STEM in schools in her spare time and received the Robert Perrin Medal from the Institute of Materials, Minerals, and Mining – all before her 30th birthday.
Rhys is also refreshingly forthright in her views. She took the time to speak to us about everything from attitudes towards disability in academia, the problem with STEM statistics, and finding that sense of belonging in science.
Would you mind telling me about your work at the University of Manchester and the research areas that interest you most?
My research interests have always been interdisciplinary – I am a bit of a magpie when it comes to research and I get excited by projects in different areas. Luckily, being a researcher in materials science means that I can apply my knowledge and skills in a wide array of areas and industries. I have recently finished my doctoral studies looking at how carbon fibre composites are damaged during impacts, and how to toughen them while keeping composites light weight, which is particularly useful in the aerospace industry. However, I have since moved over to research in biomedical materials, specifically within tissue engineering, where I am researching biocompatible composite scaffolds for tissue regeneration.
You set up Women of Science in 2016 to share stories about real people in science. How has this been?
When I set up Women of Science, I first looked at it as a personal project that could be of use in schools to young people. However, it became apparent fairly quickly that access to relatable role-models in STEM was needed, not just in schools but also for women across the STEM industry.
Since then, we have been fortunate to be awarded funding to grow the work we do and expand our audiences. One of the most important actions I have taken with Women of Science is to set up an advisory board (which includes a diverse range of women) to share ideas and to influence the direction and activities of Women of Science.
As well as the impact on others, Women of Science has had a huge impact on me personally. When I set up Women of Science I was going through a difficult period of feeling isolated, and found it difficult to feel a sense of belonging in science and in research. By reaching out and hearing other women’s stories – not just their achievements, but also their doubts, worries, and difficulties – I found that I did belong in STEM. I just had to search for it.
Would you mind sharing some of the successes and challenges you’ve experienced in your own career?
At 29, towards the end of my PhD, I was diagnosed as autistic. Looking back, I can see that the challenges I faced, particularly because of depression, anxiety, and isolation, were due to my needs not being considered or met. Being disabled in academia is an ongoing challenge. It is still a fight to gain equitable working arrangements, opportunities, and acceptance.
However, I can also see how the successes I have had, such as setting up Women of Science, and being a part of other projects are a result of ‘being different’. My strongest quality is a diversity of perspective and experience and an eagerness to be a part of a range of different projects.
>> We’re keen to hear diverse perspectives from people working in the chemical industry. Get in touch with us at: eoin.redahan@soci.org
You have championed inclusivity in STEM. Do you think academic institutions and other workplaces could be more inclusive?
Yes. I think there is a huge amount of awareness and conversation about inclusivity in academia and industry, but not nearly as much action and intervention. Often I see workplaces with inclusive policies, but with little consideration of monitoring, evaluating, or reconsidering those policies. We must move past equity, diversity, and inclusivity being a checkbox exercise. The issues faced by women in the workplace are intersectional and complex, and so require well considered, complex solutions.
According to WISE, women now make up 24% of the STEM workforce in the UK. It estimates that this number could rise to 29% by 2030. What do you think about these figures?
While the number of women in STEM is a common metric when considering equality, this does not accurately portray issues surrounding inclusion and belonging. How are women treated? Do they have the opportunity to advance? Are there equitable policies and measures in place? This is particularly true of women in STEM who identify with other protected characteristics around race, disability, sexual orientation, and class. Once you dig into the statistics (where available) further, it is clear that the numbers given are not sufficient to describe the current situation for all women in STEM.
Also, the ‘leaky pipeline’ model is often considered, that is, that the number of women in STEM fall as we follow the statistics from school, to university, and onto the workplace. However, what is not always considered is that, as with a leaky pipeline, when more women are added, rather than ‘fixing’ the pipeline, the cracks become more obvious. Eventually, we reach a point when the pipeline is fractured. We must focus on repairing these cracks, not just increasing a numerical metric.
Additionally, in this current climate, it is incredibly difficult to make predictions as to what the future holds for the number of women in the STEM workforce. A couple of years ago, we could not foresee the impact that a global pandemic would have on women. When we consider the possible effects of climate change over the next decade, can we predict the burden that will be placed on women, or how this will affect women’s choices?
What’s next for you? Are you involved in any exciting projects?
With Women of Science, we have three projects that will be launched towards the end of the year, including a new website, flashcard activities for young people, and a report on the impact of the pandemic on women in STEM. Further ahead, I would love to expand the reach of Women of Science further, working with podcasting and film, as well as reaching out to policy makers. Personally, I am excited to get my teeth stuck into a new research project and see where that leads, as well as doing more teaching, consulting, and any other opportunities that come my way!
>> Are you interested in getting involved in Women of Science? Visit: www.womenofsci.com
A little talked about element, with the atomic mass 140, plays a surprisingly important role in everyday life. It has not only lit many a path, but can be credited with improving and saving the lives of billions of people by enabling cleaner air.
In his talk '140Ce: White light & Clean Air' Andy Walker, Johnson Matthey’s Technical Marketing Director explained why the soft, ductile silvery-white metal Cerium, deserves more recognition.
Walker began by outlining the history of SCI, celebrating its 140th anniversary this year. As an employee of Johnson Matthey, Walker highlighted that George Matthey was among the pioneers of SCI. In addition Walker explained that his PhD research had involved looking at catalysts that included Cerium.
Cerium is a lanthanide and the 26th most abundant element on earth. Indeed it was the first lanthanide to be discovered, found as its ore cerium silicate, in 1803. Cerium makes up 66ppm of the earth’s crust, which is about 5 times as much as lead. It is the only one of the lanthanides able to take on the +4 oxidation state, making it very useful in some of its applications. It is mined in the US, Brazil, India, Sri Lanka, Australian and China, with annual global production of 24 000 tonnes.
However, this straightforward look at the history of Cerium conceals a much more interesting narrative about how this element shaped the life of a number of prominent chemists of the day. Indeed Cerium was found as early as 1751 at a mine in Vestmanland, Sweden by Axel Cronstedt, who also discovered Nickel. Believing it to be an ore of Tungsten, he sent it to Carl Wilhelm Scheele for analysis. However, Scheele was not able to identify it as a new element.
This turn of events for Scheele, perhaps unfairly, helped to seal his moniker as the ‘unlucky chemist’. Scheele, a prominent chemist and pharmacist, had a number of discoveries to his name. He isolated lactic acid, and discovered hydrogen fluoride and hydrogen sulphide.
But as Walker explained, his most notable discovery was oxygen, some three years before Joseph Priestley. Sadly for Scheele; it took him six years to publish his findings, by which time Priestley had already presented his data. Putting a contemporary slant on Scheele’s misfortune, Walker added that the cautionary tale here was that getting things out into the public domain as soon as possible can be important to ensure credit goes to the right people.
Further work by Scheele led to the discovery of a number of elements including barium and chlorine, but sadly he did not receive any recognition because he didn’t manage to isolate them and identify them correctly. The chemist Sir Humphrey Davy did so, some years later, getting the credit for their discovery and isolation.
So it was in 1803 that chemists Wilhelm Hisinger and Jons Jacob Bezelius proved that Cerium was indeed a new element, naming it Cerium after an asteroid/dwarf planet which had been called Ceres. The successful isolation of Cerium took place in 1875, carried out by American chemists William Hillebrand and Thomas Norton, by passing an electric current through molten cerium chloride.
99.95% fine cerium isolated on white background
Once isolated, the earliest application of Cerium was in incandescent gas mantles. Developed by Carl Auer von Welsbach, in 1891, he perfected a mixture of 99% thorium oxide and 1% ceria, which gave a soft white light. Introducing his new mantle commercially in 1892, von Welsbach was able to monetise his development selling his product throughout Europe.
Gas mantles have been replaced, but Cerium’s importance in producing white light remains. As Walker explained, most white LEDs use a blue gallium nitride LED covered by a yellowish phosphor coating made of cerium-doped Yttrium Aluminium Garnet crystals.
In the medical arena, Cerium was used by Sir James Young Simpson, Professor of Medicine and Midwifery at Edinburgh who did a lot of work in the area of anaesthetics. Simpson found that cerium nitrate suppressed vomiting, particularly that associated with morning sickness, and well into the last century, medication containing Cerium could be bought over the counter. In addition Cerium has been the basis of treatments for burns.
Other applications for this versatile element are self cleaning ovens and mischmetal alloy, used in flints for cigarette lighters. Walker shared that the chemist and author Primo Levi, while imprisoned in Auschwitz, was able to steal cerium-iron rods from the laboratory he was forced to work in. Making them into cigarette lighter flints, he was able to barter for bread. Cerium is used to harden surfaces; it is a good polishing agent. Cerium sulphide has been used to replace the pigment cadmium red as a non-toxic alternative and Cerium is widely used across the chemical industry as a catalyst to produce a host of chemicals.
Catalysis is probably where Cerium has impacted most people as the element is the basis for the catalytic converters that have provided cleaner air for billions of people. Walker explained that the driver for the development came during the 1950s when photochemical smog was a problem in the Los Angeles Basin. Measurements at the time indicated that vehicles were responsible for the majority of the hydrocarbon and NOx emissions that led to the polluted air.
This turn of events led researchers to develop systems that could mitigate the emissions. Johnson Matthey was among those doing the early work on catalytic converters. Meanwhile, the automotive industry was pushing back on their introduction, concerned about the costs, durability and effectiveness. Working with Ricardo Engineering, Johnson Matthey carried out durability tests over 25 000 miles which also showed that the catalysts could pass US emissions tests.
The catalysts had to operate in three ways, at the same time, oxidising carbon monoxide (CO) and hydrocarbons (HC) while reducing NOx. Early catalysts, circa 1975, were based on Palladium and Platinum and focused on oxidising the CO and HC. Around 1978 a second catalyst was introduced to reduce NOx.
However, the introduction of Cerium then made it possible to develop a single catalyst that was able to carry out the functions that the researchers had wanted to achieve. Hence, 1981 saw the introduction of the three way catalytic converter with all three reactions enabled over a single catalyst. More recently ceria-zirconia oxide based catalysts have been developed with much higher oxygen storage capacity than ceria.
The impact of these developments has allowed the implementation of much more stringent air quality and emissions standards. Indeed Johnson Matthey estimates that its Cerium-based catalysts are responsible for removing around 40 tonnes of pollutants every minute of every day.
A single element has indeed impacted many lives.
Antimicrobial resistance (AMR), now referred to as the silent pandemic, is causing governments, regulatory and health bodies to make a lot of noise.
Issuing a statement in late August 2021, the Global Leaders Group on Antimicrobial Resistance called on countries to ‘significantly reduce the levels of antimicrobial drugs used in global food systems’. The Global Leaders Group on Antimicrobial Resistance includes heads of state, government ministers and leaders from the private sector and civil society. It was established during 2020 to accelerate global political momentum, leadership and action AMR.
Co-chaired by Mia Amor Mottley, Prime Minister of Barbados and Sheikh Hasina, Prime Minister of Bangladesh, the Group is calling for all countries to take action to tackle the issue. Steps include: Ending the use of antimicrobial drugs that are of critical importance to human medicine to promote growth in animals, eliminating or significantly reducing over-the-counter-sales of antimicrobial drugs that are important for medical of veterinary purposes, and reducing the overall need for antimicrobial drugs by improving infection prevention and control, hygiene, bio security and vaccination programmes in agriculture and aquaculture.
Leaders are calling for the reduction in the use of antimicrobial drugs.
Speaking at the second meeting of the Global Leaders Group on Antimicrobial Resistance, Inger Andersen, Under-Secretary-General of the United Nations and Executive Director the United Nations Environment Programme said: ‘Already 700 000 people die each year of resistant infections. There are also serious financial consequences: in the EU alone, AMR costs an estimated €1.5 billion per year in health care and productivity costs…’ But Andersen added that now was an opportune moment to make change. ‘With concern over zoonotic diseases at an all-time high, governments can take advantage of the synergies available from tackling emerging disease threats concurrently. The Global Leaders Group has strategic access to forums to promote AMR integration in post-covid-19 plans and financing…It’s time to for us to act on the science and respond rapidly to AMR,’ Andersen said.
The Communiqué from the G7 Health Ministers’ Meeting held in Oxford, UK during June also gave significant space the AMR issue and the link with the pandemic. ‘We reiterate the need for ongoing education and reinforced stewardship of the use of antimicrobials, including avoiding their use where there is no science-based evidence of effectiveness. The pandemic has also highlighted the importance of infection prevention and control measures to tackle AMR, targeting both health-care associated and community-associated infections.’ Adding a sense of urgency the Communiqué continued: ‘We must act strongly and across disciplines if we are to curb the silent pandemic of antimicrobial resistance.’
A letter from the BactiVac Bacterial Vaccinology Network reminded the G7 Health Ministers that the 2016 O’Neill Report estimated that by 2050, 10 million lives each year and a cumulative US$100 trillion of economic output will be at risk due to increasing AMR unless proactive solution are developed now. In its letter to the G7, the Network issued this warning. ‘The headlines on AMR may have less immediate impact, but the news is no less stark. Over the long-term, AMR bacteria will cause more prolonged suffering than covid-19, with a more insidious impact on all our lives.’ Signatories to the letter included Professor Calman MacLennan, Senior Clinical Fellow and Group Leader, Jenner Institute, University of Oxford, Professor of Vaccine Immunology, University of Birmingham.
Researchers are collaborating to understand how AMR is impacted by a range of factors
The G7 also stressed the need for collaborative efforts for a better understanding of how AMR is impacted by a range of factors. Taking up this challenge; several initiatives has been put in place to study this. Most recently the United Nations Environment Programme and the Indian Council of Medical Research have launched a project looking at ‘Priorities for the Environmental Dimension of Antimicrobial Resistance in India.’ The project aims to strengthen the environmental aspects of national and state-level AMR strategies and action plans. In a similar development the European Food Safety Agency published an assessment of the role played by food production and its environment in the emergence and spread of antimicrobial resistance. Publishing the findings in the EFSA journal, the report indicated that fertilisers of faecal origin, irrigation and water are the most significant sources of AMR in plant-based food production and aquaculture.
Meanwhile, the first quarter of 2021 saw Ineos donate £100 million to the University of Oxford to establish a new antimicrobials research facility. The Ineos Oxford Institute for Antimicrobial Resistance aims to create collaborative and cross disciplinary links involving the university’s department of chemistry and department of zoology. The Institute also intends to partner with other global leaders in the field of AMR.
Partnering with India, the UK has committed £4 million to the AMR fight. With a total investment of £8 million, the partners have established five joint research projects which aim to develop a better understanding of how waste from antimicrobial manufacturing could be inadvertently fuelling AMR.
Sarah Davidson has made impressive strides in a short space of time. She has risen to Group Sustainability Coordinator for global Research and Technology at speciality chemicals firm Croda and won the Young Ambassador Award at this year’s Chemical Industry Awards.
In the first blog in our Women in Chemistry series, we caught up with Sarah for a chat on embedding sustainability in the workplace, the need for more diversity in senior roles, and the best bit of advice she received.

Tell us about your career to date.
I loved chemistry at school, so I started off by doing a Master’s in Chemistry at the University of Sheffield. During the course I did a placement year, which was my first taste of working in industry. Once I finished my degree, I was torn between staying in academia and doing a PhD or going into industry. I chose to go into industry because I had enjoyed my placement year so much and saw where I could make an impact.
I was accepted onto Croda’s Graduate Development programme, where I had three placements around the business. Croda is a speciality chemicals company, so my placements included working as an applications scientist and synthetic chemist. However, it was my placement working with the Sustainability team that I loved the most.
After the Grad Scheme I became Group Sustainability Coordinator for Global R&D. This combined my experience in R&D and sustainability in a brand role that didn’t exist in Croda before. This role allows me to use my technical knowledge and understanding of the way the global team works to enable those responsible for Croda’s new product innovations to include sustainability as an integral pillar in new product development.
What does your day-to-day role involve?
In my role, my main focus is on getting our scientists to think about sustainability during product and process development. At a fundamental level this requires me to change their mindsets around sustainability, getting them to see it is important to what we do and understand what it means.
To do this, I have developed a number of tools including checklists, clearly defined procedures and training documents. I have been working to get these new procedures adopted over the global R&D team by fitting them into existing protocols. Another part of my role is to support our corporate targets and I am part of a number of working groups to do this.
One working group looks at how we define a consistent methodology for Life Cycle Assessments or LCA. In this group I have been doing research to understand the current methods around LCA, and what our customers want in terms of sustainability data. I also help gather data to show where we are up to with these goals, so we understand what actions we need to take to move forward. On a day-to-day basis I will have meetings to discuss the projects I am involved in, conduct research and reach out to other teams and functions to see what they are working on too.
Which aspects of your job motivate you most?
For me sustainability is the future, not only for the chemical industry but for the world. Knowing that I am having a positive impact on sustainability in my role is what motivates me the most. I try to live a sustainable life, and what I do at work is just an extension of that.
What personal challenges have you faced and how have you overcome them?
To embed sustainability into our ways of working, I need to change people’s mindsets, and subsequently their behaviour. Seeing this change in people is incredibly rewarding. However, it is also one of the biggest challenges. Some of our teams have been working in the same roles for decades without any change. So, it is my job to make these changes easier for them to adopt and persuade them of the benefits in doing so. To overcome this challenge, I have had to work on my influencing skills and know what will work with the audience I am speaking to.
What is the greatest future challenge for people in your industry and how could this be addressed.
Sustainability, and addressing the issues we face as a result of climate change, are some of the biggest challenges we will face as an industry. We are in a lucky position that we can achieve a competitive advantage with sustainability, but our main goal is to protect our planet. This gives us a big opportunity for collaboration where we may not have had one before. I think we can only solve this challenge by collaborating across the supply chain, across country borders, and between industry and academia.

>> Not everyone takes the standard career path into chemistry. Take a read of Claudio Laurenco’s unusual, inspiring story.
Which mentors have helped you along the way and how did they make a difference?
I feel like I have a long list of mentors and am very lucky to be able to call on so many people for advice. The best thing I have learnt from them is to pursue what I enjoy most, as people will be able to see my passion. This will help me move forward in my career. Having mentors who have confidence in me and my ability has helped me build my own confidence, something which I can lack from time to time. My mentors are great sounding boards for ideas, whether that is to do with things I want to try in my job or on the direction of my career.

What is the current state of play within your sector with respect to equality, diversity, and inclusion – and is enough being done to attract and retain diverse talent?
I don’t think so. We need to do more to attract and retain diverse talent. We seem to be relatively diverse and inclusive at an academic level, which disappears in industry. There must be a reason for this. There may be bias within recruitment processes, or within job descriptions for senior roles, which means there is less diversity as you move up in organisations. We need to make sure that there are equal opportunities within industry for everyone and make sure everyone has a path to progression that works for them.
Is there any advice you would give to young professionals starting out in your area, especially young women?
Understand where you are different and use that as your advantage. Everyone has a unique lived experience that they bring with them into all situations. As women we have a different perspective to men. This doesn’t mean it is less valuable, it is just different. When you feel like you are in a minority as a woman, or are not being listened to, it is important to remember that our opinions are equal regardless of our background, gender or ethnicity. You have the same right to share your views, as the majority do theirs.
>> We’re always keen to hear from women who are making a real difference in chemistry. If you know someone who you think we should cover, please get in touch with us at: eoin.redahan@soci.org.
SCI was pleased to support #BlackInChem, working alongside our Corporate Partners and members to amplify the voices of our Black chemists.
We have heard stories from several Black chemists who highlighted the steps being taken by many companies to increase diversity. But we can also see that there are many more steps that can be taken to encourage the next generation of budding Black chemists and scientists.
#BlackInChem has had support from Scott Bader, an SCI Corporate Partner, with both Damilola Adebayo and Luyanda Mbongwa sharing their perspectives as employees of Scott Bader. Elsewhere, Cláudio Laurenço gave a compelling account of his journey to become a post-doctoral research associate at a leading consumer goods company.
Cláudio Laurenço worked for free and was overlooked before eventually securing his PhD and starting his career in chemistry.
These chemists are following in the footsteps of some pioneering Black scientists such as Percy Lavone Julian, who has been profiled on the SCI Blog.
Many organisations have expressed their support and shared thoughts on what steps they are taking to encourage and ensure diversity. Indeed, #BlackInChem is a global effort and companies such as GSK have shown their support as well as numerous Black chemists talking about their experiences and achievements over the last week.
Percy Lavon Julian’s pioneering work enabled a step-change in the treatment of glaucoma | Editorial credit: spatuletail / Shutterstock.com
Over the coming months, we will be profiling other Black chemists, past and present, and continuing the dialogue around diversity.
>> We’re always keen to hear about diverse perspectives within chemistry. If you’d like to share your story, please contact: muriel.cozier@soci.org or eoin.redahan@soci.org.
For Cláudio Lourenço, the path from student to multidisciplinary scientist has been far from smooth. The Postdoctoral Research Associate reflects on the institutional challenges that almost made him give up, the mentor whose support was so important, and the barriers that block the way for young Black chemists.
Please give a brief outline of your role.
I work for a leading consumer goods company. I am a multi-disciplinary scientist contributing to the development of novel formulations for household products.
Why are you supporting #BlackInChem?
I’m supporting #BlackInChem because I am a champion for diversity. I believe that what we see from our windows in the street is what we must have inside our workplaces. In an ideal world we should all have the same opportunities, but unfortunately this is somehow far from the truth. We need to motivate our young Black chemists to aim for a career in science by providing welcoming environments and real opportunities instead of just ticking boxes. We need to showcase our Black chemists to show to the younger generation that they can also be one of us.
What was it that led you to study chemistry and ultimately develop a career in this field? Was this your first choice?
I have always been passionate about research and science. My father had a pharmacy, so I was always close to chemistry and was a very curious child. Yes, it was my first choice but the lack of opportunities and trust from universities and scholarship providers made it a long run. My motivation faded and I nearly gave up.
Was there any one person or group of people who had a specific impact on your decision to pursue your career path?
Yes, but after my degree I nearly gave up. It took me nearly two years and changing cities to find something (a voluntary position). I was always keen on taking up mentors to show me how to progress in my career. There were a few people who helped me by training me and teaching me how to navigate the scientific world and pursue a career in science.
I only got my first job (which I worked for free) because of Peter Stambrook, an American scholar from the University of Cincinnati, who I met through a friend while polishing glasses in a restaurant. This man was open and keen to put a word in for me at a leading university in the UK. He taught me so much on how to be a scientist and humbly grow up and make a career in science. Eventually, all his advice kept me on the right path.
What impact would you like to see #BlackInChem have over the coming year?
More Black students in postgraduate courses and an increase in role models to motivate the younger generations to pursue careers in chemistry.
Could you outline the route that you took to get to where you are now, and how you were supported?
Personally, my career path was far from easy. I only managed to get my PhD at 38 years of age. I needed to first prove myself. Despite all my efforts and dozens of applications, I was never considered a good candidate. I needed to work for free for two years to land a proper job in my field of choice. During that time I took on many odd jobs to support myself. I worked for a top 10 university for free and they never saw my worth or gave me an opportunity. With that experience I landed a proper job at a leading pharmaceutical company. After one year with them, they funded my PhD studies and now here I am with a career in science.
Considering your own career route, what message do you have for people who would like to follow in your footsteps?
Never ever give up - it is possible. Look for the right mentors and be humble. You do not need to reinvent the wheel, but only to find someone who can lend you theirs. Learn to grow from the experiences of others and be ready to fail a couple of times - we all do. Be open to learn and never be afraid of following your dreams.
>> At SCI, we’re proud to support #BlackinChem. Reach out to us with your stories.
What do you think are the specific barriers that might be preventing young black people from pursuing chemistry/science?
I think one of the biggest barriers that prevent people from pursuing careers in science is the lack of role models. If we only show advertisements for chemistry degrees with White people, it’s not encouraging for Black students to pursue a career there. The same goes for when we visit universities; role models are needed. No one wants to be the only Black person in the department. Universities need to embrace diversity at all levels. I understand that tradition sometimes prevents this, but we need to change and ignore tradition for a bit.
What steps do you think can be taken by academia and businesses to increase the number of Black people studying and pursuing chemistry/science as a career?
Showcase Black chemists and inventors to motivate the younger generations and show society that Black people are not only artists and musicians. Target extracurricular activities in schools where children are from disadvantaged backgrounds. Train your staff to be open. Create cultural events that not only target Black people but also for other people to learn and see that in the end we are all equal. We all need to learn to embrace our differences and grow together.
>> As we celebrate #BlackinChem, we mark the achievements of some inspirational chemists. Read more about the amazing career of Percy Lavon Julian.
If you’re a vegan, do you really want to eat a ruby-red slab of plant protein that looks like lamb? If you are a health obsessive, would you opt for an ultra-processed, plant-based product if you knew it didn’t contain many vitamins and micro-nutrients? And why, oh why, are we so obsessed with recreating the taste and appearance of the humble hamburger?
These questions and more were posed by Dr David Baines in the recent ‘No meat and two veg – the chemistry challenges facing the flavouring of vegan foods’ webinar organised by SCI’s Food Group. The flavourist, who owns his own food consultancy and is visiting Professor at the University of Reading, painted a vivid picture of our changing culinary landscape – one in which 79% of Millennials regularly eat meat alternatives.
And this shift in diet isn’t just the preserve of the young. According to Dr Baines, 54% of Americans and 39% of Chinese people have included more plant-based foods and less meat in their diets. Furthermore, 75% of Baby Boomers – those born between 1946 and 1964 – are open to trying cultivated meat.
There are many reasons for this gradual shift. The woman biting into Greggs’ famous vegan sausage roll and the woman who carefully crafts her bean burger may have different reasons for choosing meat alternatives. For some, it’s an ethical choice. For others, it’s environmental or health-related. And then there are those of us who are simply curious.
Pea protein powder is used in plant-based meat alternatives.
Either way it’s an industry that, if you’ll excuse the pun, is set to mushroom. According to Boston Consulting Group and Blue Horizon research, the global meat-free sector will be worth US$290 billion by 2035. They also claim Europe will reach peak meat consumption by 2025, and Unilever is aiming to sell US$1 billion-worth of plant-based meat and dairy alternatives by 2025-27.
In his entertaining talk, Dr Baines outlined the extrusion processes that turn wheat and pea proteins into large ropes of fibrous material and how soy isolates are spun into textured proteins using looms like those used in the cotton industry. He explained how calcium is used to imitate the chewable texture of chicken and how Impossible Foods is using the root nodules of bean plants to produce the red colour we recognise so readily in meat.
>> For more interesting SCI webinars on battery developments, medicinal chemistry and more, check out our events page.
So, how close are we to products with the appearance, taste and texture of, let’s say, beef? ‘I think that will come from cultured meat to start with,’ he said. ‘Where the protein is produced, it will still need to be flavoured, but the fibres will have formed and the texture is already present in some of those products.
‘It’s a big ask and it’s been asked for a long time. It’s going to be a long time before you put a piece of steak on one plate and a plant-based [product] on another and they will be visually, texturally and taste(-wise] identical.’
And what appetite do people even have for these plant-based facsimiles? ‘There are people who want plant proteins not to look like meat, and there are people who want them to look like meat,’ he added. ‘The driver at the moment is to make them look like meat, and the driver is to make it taste like meat too.’
Baines wondered aloud about the bizarre fixation some have with recreating and eating foods that look and taste like beef burgers. In contrast, he pointed to the examples of tofu and soy-based products that have been developed in South East Asia – distinct foods that do not serve as meat substitutes.
Plant-based proteins are undoubtedly part of our culinary future, but these products have other barriers to surmount beyond taste and texture. There is no getting around the fact that plant-based proteins are ultra-processed in a time when many are side-stepping processed foods. Baines also explained that these protein- and fibre-rich foods tend to have lower calorific content, but lack vitamins and micronutrients. ‘Will they be supplemented?’ Baines asked. ‘How much will the manufacturers of these new products start to improve the nutritional delivery of these products?’
We have now entered the age of the gluten-free, vegan sausage roll.
But it’s easy to forget that the leaps made in recent years have been extraordinary. Who would have predicted back in 1997 – when Linda McCartney was at the vanguard of the niche, plant-based meat alternative – that a vegan sausage roll would capture the imaginations of a meat-hungry nation? Who would have foreseen fast-food manufacturers falling over each other to launch plant-based burgers and invest in lab-grown meat?
As Dr Baines said: “This is a movement that is not going away.”
>> Our soils provide 97% of our food. Read more about how they are undervalued and overused here.
This week SCI is joining with business and academia to mark #BlackInChem, an initiative to advance and promote a new generation of Black chemists.
Over the coming weeks, we shall be profiling past and present Black chemists, many of whom are unsung heroes, and whose work established the foundations on which some of our modern science is built. We start with the outstanding contribution made by Percy Lavon Julian (1899-1975).
Born on 11 April 1899 in Montgomery, Alabama, US, Percy L Julian was the son of a clerk at the United State Post Office and a teacher. He did well at school, and even though there were no public high schools for African Americans in Montgomery, he was accepted at DePauw University, Indiana, in 1916.
Due to segregation Julian had to live off campus, even struggling initially to find somewhere that would serve him food. As well as completing his studies, he worked to pay his college expenses. Excelling in his studies, he graduated with a BA in 1920.
Julian wanted to study chemistry, but with little encouragement to continue his education, based on the fact there were few job opportunities, he found a position as a chemistry instructor at Fisk University, Nashville, Tennessee.
In 1922 Julian won an Austin Fellowship to Harvard University and received his MA in 1923. With no job offers forthcoming, he served on the staff of predominantly Black colleges, first at West Virginia State College and in 1928 as head of the department of chemistry at Howard University.
In 1929 Julian received a Rockefeller Foundation grant and the chance to earn his doctorate in chemistry. He studied natural products chemistry with Ernst Späth, an Austrian chemist, at the University of Vienna and received his PhD in 1931. He returned to Howard University, but it is said that internal politics forced him to leave.
Physostigmine was synthesised by Julian
Julian returned to DePauw University as a research fellow during 1933. Collaborating with fellow chemist and friend Josef Pikl, he completed research, in 1935, that resulted in the synthesis of physostigmine. His work was published in the Journal of the American Chemical Society.
Physostigmine, an alkaloid, was only available from its natural source, the Calabar bean, the seed of a leguminous plant native to tropical Africa. Julian’s research and synthesis process made the chemical readily available for the treatment of glaucoma. It is said that this development was the most significant chemical research publication to come from DePauw.
Calabar bean
Once the grant funding had expired, and despite efforts of those who championed his work, the Board of Trustees at DePauw would not allow Julian to be promoted to teaching staff. He left to pursue a distinguished career in industry. It is said that he was denied one particular position as a town law forbid ‘housing of a Negro overnight.’ Other companies are also said to have rejected him because of his race.
However, in 1936 he was offered a position as director of research for soya products at Glidden in Chicago. Over the next 18 years, the results of his soybean protein research produced numerous patents and successful products for Glidden. These included a paper coating and a fire-retardant foam used widely in World War II to extinguish gasoline fires. Julian’s biomedical research made it possible to produce large quantities of synthetic progesterone and hydrocortisone at low cost.
Percy Lavon Julian | Editorial credit: spatuletail / Shutterstock.com
By 1953 Julian Laboratories had been established, an enterprise that he went on to sell for more than $2 million in 1961. He then established the Julian Research Institute, a non-profit research organisation. In 1967 he was appointed to the DePauw University Board of Trustees, and in 1973 he was elected to the National Academy of Sciences, the second African American to receive the honour.
He was also widely recognised as a steadfast advocate of human rights. Julian continued his private research studies and served as a consultant to major pharmaceutical companies until his death on 19 April 1975. Percy Lavon Julian is commemorated at DePauw University with the Percy L Julian Science and Mathematics Center named in his honour. During 1993 the United States Postal Service commemorated Julian on a stamp in recognition of his extraordinary contribution to science and society.
SCI has selected Harriet McNicholl from AstraZeneca as the 2021 National Undergraduate Placement Student of the Year.
The national undergraduate placement symposium brings together chemistry students undertaking industrial research placements each year. Students working in organic, biological, supramolecular, physical organic, medicinal chemistry and related fields are invited to submit posters. The finalists are then selected to present orally at the virtual symposium. This year’s applicants included students from organisations such as AstraZeneca, GlaxoSmithKline, UCB, Syngenta, Charles River and more.
Harriet McNicholl’s chemistry will be used to manufacture drug products to support patients in phase-II clinical trials.
As part of this symposium, Harriet McNicholl from AstraZeneca was invited to present her research to develop a safe, inexpensive and commercially viable process towards AZD5991, a candidate therapeutic for the treatment of acute myeloid leukaemia.
Encapsulating AstraZeneca’s dynamic and data driven approach to turning molecules into medicines, Harriet highlighted how the SELECT criteria, automation and High Throughput Experimentation were used to design and optimise a process. Harriet’s work aimed to maximise efficiency and sustainability, and her chemistry will be used to manufacture drug products to support patients in phase-II clinical trials.
Harriet is in the third year of her chemistry integrated Master’s degree (MChem) at the University of Liverpool and is currently undertaking a synthetic chemistry industrial placement within Chemical Development (CD) at Macclesfield.
‘I have thoroughly enjoyed my placement year within Chemical Development at AstraZeneca,’ she said. ‘It has been incredibly rewarding knowing the science I’ve worked on has the potential to fundamentally transform oncology patients’ lives. This opportunity has enabled me to develop many of my technical and soft skills and motivated me to pursue a career within the pharmaceutical industry.’
Dave Ennis, Vice President of Chemical Development for AstraZeneca in Macclesfield, said: ‘Congratulations to Harriet who has made significant contributions to our development activities in Chemical Development. It is a reflection of the quality of students we attract to our sandwich student programme; I’m proud that we give our students a great insight to drug development by being active participants in our projects, and it is highly motivating for our scientists in helping to coach and develop others - a win-win for all involved.
‘Over the past 25 years, we have had a successful rolling programme of sandwich students from a variety of universities that has helped to attract the next generation of scientific talent to AstraZeneca and the wider industry. Looking forward to our next cohort in 2021, and I’m sure they will compete for the prize next year’.
Harriet’s poster submission
Dr Andrew Carnell, Director of Year in Industry Courses at the Department of Chemistry in the University of Liverpool, added: ‘I am delighted that Harriet has been awarded this prestigious prize for her work during her placement at AstraZeneca. She is a credit to the department and to the university. Our Year in Industry students gain a huge amount from their placements, not only in terms of practical experience and technical knowledge but increased confidence and employability. Students return to us highly motivated for their final year and often go on to secure excellent and rewarding positions in today’s competitive job market.’
As part of this event, keynote speaker James Douglas (Manager of AstraZeneca’s Catalysis, High Throughput and Synthesis Technologies team) noted that his career journey started with a placement year at GlaxoSmithKline in Stevenage. James went on to describe the benefits of doing a placement year and how the skills he gained from his year in industry helped him to secure a Ph.D. at the University of St Andrews and a postdoctoral position with Eli Lilly in the United States.
This year’s competition featured many strong entries. Congratulations to runners up Daniella Hares (AstraZeneca, University of Southampton) for her presentation outlining computational techniques for drug discovery and poster prize winner Jake Odger (Sosei Heptares, University of York). The competition was hosted and organised by the Society of Chemical Industry Young Chemists’ Panel
For more on this year’s National Undergraduate Placement Student of the Year competition, visit: https://istry.co.uk/postercompetition/4/
From genome mining and green synthesis, to tackling tuberculosis and computational methods to help cure malaria, the chemists of tomorrow have been busy showcasing their talents as part of the Society of Chemical Industry Young Chemists Panel’s National Undergraduate Online Poster Competition 2021.
A snapshot of these students’ talents is bottled below in their own words. So, which one of these 15 entries do you think contains the most potential?
1: Genome mining for myxobacterial natural products discovery
Emmanuelle Acs et al., University of Glasgow
Natural products have always had a privileged place in drug development programmes, but their discovery is long and tedious. Genome mining arises as a solution allowing the finding of compounds never seen before. Using an array of bioinformatic softwares, the myxobacterial genome was explored for new Ribosomally and Post-Translationally modified Peptides (RIPPs). Myxobacteria are soil-dwelling bacteria known for the number of secondary metabolites they produce, and they have proven to hide many more within their genome. Indeed, our analyses have led to the potential discovery of nine new myxobacterial natural products. The nature and class of these products is to be confirmed by biosynthesis in the laboratory.
2: De novo design of a lanthanide-binding peptide inspired by the methanol dehydrogenase enzyme active site
Olivia Baldwin et al., University of Birmingham
Lanthanides were thought to be biologically irrelevant until the discovery of bacteria containing the lanthanide-dependent methanol dehydrogenase (Ln-MDH) enzyme. There has been interest in exploiting the attractive properties of the lanthanides by the de novo design of artificial proteins, aiming to explore protein structures and functions not observed in biology. Here, a lanthanide-binding peptide, CS1-0, has been designed de novo and shown to bind to europium and pyrroloquinoline-quinone (PQQ), a key component of the Ln-MDH active site. This partial recreation of a biologically relevant lanthanide binding site is a step towards the ultimate goal of de novo design, to create functional artificial metalloproteins with simplified structures.
3: Template-directed synthesis of porphyrin nanorings: a computational and experimental study
Janko Hergenhahn et al., University of Oxford
Template-directed synthesis provides a route to achieve porphyrin nanorings by favouring ring-closure reaction over oligomerisation. A structurally new template with 12 binding sites has been proposed for the synthesis of novel porphyrin rings; however, initial unsuccessful reactions have raised questions about the binding efficiency of this template to the linear substrate. We have employed classical and quantum modelling together with experimental techniques to explore template-substrate binding in solution and shed light on this process. Titration experiments and modelling have enabled us to study the occupancy of different binding sites and quantify the influence of strain on binding, further guiding novel designs.
4: Transborylation enabled boron-catalysed hydrocyanation of enones
Kieran Benn et al., University of Edinburgh
Hydrocyanation offers an orthogonal route to synthetically ubiquitous amines. Current hydrocyanation methodologies are dominated by the use of acutely toxic hydrogen cyanide gas and transition metal catalysts. Here the application of main-group catalysis and transborylation is reported for the formal hydrocyanation of functionalised alkenes. The catalytic protocol was optimised and applied to a broad range of substrates (20 examples), including examples where chemoselectivity was demonstrated in the presence of reducible functionalities and Lewis basic groups. Mechanistic studies support a proposed catalytic cycle in which B–N/B–H transborylation was a key to catalyst turnover.
Students at the University of Glasgow have used computational analysis to help tackle malaria.
5: Investigation of the structure-property relationship between hydrophobicity and the antimicrobial activity of AMP-mimic copolymers
Xiyue Leng et al., University of Birmingham
Antimicrobial peptides are increasingly employed as new-generation antibiotics, with their amphiphilic nature (contain both hydrophobic and cationic components) mimicked by polymers to enable a more cost-effective approach. However, there is a lack of a quantitative pre-experiment indicator to provide a prospect on their potency. The overall hydrophobicity represented by LogP/SA was proposed to rapidly identify candidates in future designing to reduce synthetic efforts. We show a comparison study between two computational tools used to calculate LogP/SA: ChemBio3D and Materials Studio, in terms of the predictive power and sensitivity, followed by the synthesis of copolymers with a different cationic side chain based on the calculation results.
6: Modelling potential SARS-CoV-2 VIRTAC warheads
Mirjam-Kim Rääbis et al., University of Glasgow
Traditional small molecule therapeutics in medicinal chemistry often require high doses to inhibit the target protein, leading to issues with safety and drug resistance. Proteolysis targeting chimeras (PROTACs) are a new class of molecule that combat these issues, as they can use the body’s own protein degradation systems to degrade targets even at low drug doses. Virus-targeting chimeras (VIRTACs) can use a similar mechanism to target viral proteins. This project uses molecular docking studies to explore potential VIRTAC warheads that target the papain-like protease of SARS-CoV-2, in an attempt to find a potential treatment to COVID-19 that would, among other benefits, offer a lower risk of antiviral resistance.
7: Design, synthesis and biological evaluation of anti-tubercular small molecules
Miriam Turner et al., Newcastle University
Tuberculosis remains one of the top 10 causes of death worldwide, therefore there exists an unmet clinical need for new and improved therapeutics that tackle increasing bacterial resistance and affordability issues. Previous studies indicate N-substituted amino acid hydrazides exhibit good activity against several strains of Mtb. Ongoing structure-activity relationship studies utilising isoniazid, a variety of amino acids, and the active imidazo[1,2-a]pyridine-3-carboxy moiety of clinical candidate Q203 have also demonstrated excellent activities. Herein we report the results of our continued evaluation of this architecture, using a scaffold hopping approach to explore the potential of this pharmacophore as a new anti-tubercular drug.
8: Towards a cure for malaria: computational analysis and design of PfCLK3 inhibitors
Skye Brettell et al., University of Glasgow
Malaria continues to pose a significant challenge to humanity. Resistance to several frontline antimalarials represents a considerable threat, marking the need for new drugs with novel mechanisms of action. Kinase inhibitors represent a potential new class of antimalarials. TCMDC-135051 is a hit compound with activity against malarial kinase PfCLK3 as well as potency in liver, blood and sexual stage parasites. During this project, sequential analysis of the PfCLK3 catalytic domain identified key structural differences between the target and its human orthologs. Molecular docking studies of TCMDC-135051 analogues using GOLD then yielded potential lead compounds with predicted high affinity for the target kinase.
9: Mind the gap! Through-space electronic stabilisation of dynamic azaacene-extended helicene diimides
Matteo Albino et al., University of York
The strain-induced contortion of non-planar, chiroptically-active helicenes caused by fjord steric repulsive interactions is well known. Fjord-mediated planarisation, on the other hand, is far less common and has typically only been achieved via inherently strong covalent bond formation. Herein, I present the properties and density functional theory (DFT) analysis of electroactive aza[5]helicenes exhibiting unexpected through-space π-electronic stabilisation in the reduced states as a result of non-covalent fjord bonding effects. Computational modelling of optical spectra and aromatic-induced current densities reveal that lone pair-repulsive nitrogens in the fjord promote favourable ring currents and reversible helicene planarisation.
10: Mitochondria targeted metal-chelators as potential therapeutic agents against Parkinson’s Disease
Sam Andrew Young et al., Northumbria University
The synthesis of metal chelating molecules, specifically hydroxypyridones (HOPOs), have been identified as potential therapeutic agents for treating Parkinson’s Disease (PD) as bidentate ligands at the two oxygen donor atoms. These ligands are selective for ferric iron in the body, which is expected to stop the reduction of this iron accumulated in the brains of PD sufferers, hindering the Haber-Weiss mechanism from taking place in the mitochondria of the cell and preventing the associated degeneration of the cells. The lipophilicity of these HOPOs is vital to the process, allowing the molecule to transverse the blood-brain barrier, the addition of a triphenylphosphonium group on the HOPO is thought to increase therapeutic effect.
At Heriot Watt University, students have investigated the skin irritation potential of nanoclays using an IATA
11: Green synthesis of n-acetylcolchinol
Adelaide Lunga et al., Loughborough University
The aim of this project is to develop a short synthesis of N-acetylcolchinol using a greener and step-economical pathway. First, aldol condensation of 3-hydroxyacetophenone and 3,4,5-trimethoxybenzaldehye using ethanolic NaOH produced the respective chalcone. The product was reduced electrochemically in DMSO:MeOH (4:1) employing carbon electrodes and NEt4Cl to the saturated benzylic alcohol, which was converted to an acetamide via Ritter reaction using H2SO4 in MeCN. In the final step, the conditions were optimised to enable electrochemical oxidative coupling of the aromatic groups to give the desired N-acetylcolchinol. This novel four-steps reaction sequence avoids use of transition metal catalysts or toxic reagents.
12: Towards the synthesis of small-molecule probes to measure endosulfatase activity
Yi Xiao et al., University of Oxford
Human endosulfatases (SULFs) are enzymes on the cell surface and in the extracellular matrix that hydrolyse 6-O-sulfate on glucosamine units within heparan sulfate proteoglycans. SULFs are involved in growth and development, muscle regeneration and tumour growth via various signaling pathways, with untapped therapeutic and diagnostic potentials. However, profiling SULFs remains a challenge. Antibodies detect their presence, but do not indicate their activity state. The current activity assay is a global sulfatase assay and is not selective in a biological sample. We propose a novel small-molecule probe to profile SULF activity by exploiting the formation of 1,6-anhydrosugar, which can be potentially used in isolated proteins and in vitro.
13: Developing machine learning models to predict the Hansen solubility parameters
Alexander Pine et al., University of Greenwich
Solubility parameters are important for pharmaceutical formulations, paint formulations and new material development. There is a need to improve the accuracy of solubility calculations, and to be able to make rapid predictions of the solubility of new molecular structures. In this project, a range of Python plugins, and open-source codes have been used to develop a Lasso linear regression machine learning model to predict the Hansen solubility parameters (HSP) - δd, δp and δh, which represents dispersion forces, dipole-permanent dipole forces and hydrogen bonding respectively with the intention of making faster and more accurate prediction in solubility.
14: The cycloaddition between cyclononyne and mesyl azide
Alexander David Robertson et al., The University of Glasgow
This research considers computational modelling of a SPAAC reaction involving cyclononyne. DFT calculations were performed on the strain promoted reaction between cyclononyne and mesyl azide. Three low energy conformers of cyclononyne with Cs, C2 and C1 symmetry were found with similar energy. The transition structures for the corresponding cycloaddition with mesyl azide were found and the C2 conformer was the lowest in energy. Product structures were found leading to the identification of the thermodynamic product of the reaction. Distortion/interaction analysis showed that the cycloalkyne was already significantly pre-constrained to its reacting geometry.
15: The assessment of skin irritation potential of nanoclays using an IATA
Holly King et al., Heriot Watt university
Clays are natural nanomaterials consisting of mineral silicate layers. They have several functional uses in everyday life. An example of nanoclays that carry out a wide range of roles is smectites which include montmorillonite (MMT), bentonite and hectorite. These nanoclays can be used in cosmetics, altering their appearance and in pharmaceuticals as drug carriers and wound dressings. Integrated approach to testing and assessment (IATA) aim to collect all relevant data into one easy to understand format that can be used to group materials. Using an IATA dedicated to skin irritation/corrosion it was found that MMT was safe for use. However, hectorite was found to be toxic at high doses indicating that it is a possible irritant to the skin.
Many thanks to the sponsors of this year’s competition: GSK, AstraZeneca, TeledyneIsco. The event runs until 9 July, so let us know what you think of the entries on Twitter at #SCIPosterComp.
If you’d like to see these students’ full posters, go to: https://istry.co.uk/postercompetition/5/?date_example=2021-06-28
Which technologies will propel industry forward and give companies that competitive advantage? According to digital consultancy McKinsey Digital’s Tech Trends Index, several technologies will have a profound and disruptive impact on industries including the chemical sector. So, which ones will have the biggest effect on the way you work in the coming decade?
1: Automation
By 2025, more than 50 billion devices around the world will be connected to the Industrial Internet of Things (IIOT) and about 600,000 industrial robots a year will be in place from 2022. The combination of these, along with industrial processes such as 3D and 4D printing, will speed up processing and improve operational efficiency.
According to McKinsey, 50% of today’s work practices could be automated by 2022 as ever more intelligent robots (in physical and software form) increase production and reduce lead times. So, how does this change look in the real world?
According to the McKinsey Tech Trends Index, 10% of today’s manufacturing processes will be replaced by additive manufacturing by 2030.
According to the Tech Trends Index, one large manufacturer has used collaborative robots mounted on automatic guided vehicles to load pallets without human involvement, while an automotive manufacturer has used IIOT to connect 122 factories and 500 warehouses around the world to optimise manufacturing and logistics, consolidate real-time data, and boost machine learning throughput.
2: Next generation computing
An almost incredible 368,000 patents were granted in next generation computing in 2020. Advanced computing will speed up the processing of reams of data to optimise research and cut development times for those in the chemicals and pharmaceuticals industries, accelerate the use of autonomous vehicles, and reduce the barriers to industry for many eager entrants.
‘Next-generation computing enables further democratisation of AI-driven services, radically fast development cycles, and lower barriers of entry across industries,’ the index notes. ‘It promises to disrupt parts of the value chain and reshape the skills needed (such as automated trading replacing traders and chemical simulations, reducing the need for experiments).’
According to McKinsey, AI will also be applied to molecule-level simulation to reduce the empirical expertise and testing needed. This could disrupt the materials, chemicals, and pharmaceuticals industries and lead to highly personalised products, especially in medicine.
3: The Bio-revolution
It doesn’t take much investigation before you realise that the bio-revolution has already begun. Targeted drug delivery and smart watches that analyse your sweat are just two ways we’re seeing significant change.
The Tech Trends Index claims the confluence of biological science and the rapid development of AI and automation are giving rise to a revolution that will lead to significant change in agriculture, health, energy and other industries.
In the health industry, it seems we are entering the age of hyper-personalisation. The Index notes that: ‘New markets may emerge, such as genetics-based recommendations for nutrition, even as rapid innovation in DNA sequencing leads ever further into hyper personalised medicine.’ One example of this at work in the agri-food industry is Trace Genomics’ profiling of soil microbiomes to interpret health and disease-risk indicators in farming.
4: Advanced materials
It’s no secret that we will need to develop lighter materials for transport, and others that have a lighter footprint on our planet. According to McKinsey, next generation materials will enhance the performance of products in pharma, energy, transportation, health, and manufacturing.
For example, molybdenum disulfide nanoparticles are being used in flexible electronics, and graphene is driving the development of 2D semiconductors. Computational materials science is another area of extraordinary potential. McKinsey explains: ‘More new materials are on the way as computational-materials science combines computing power and associated machine-learning methods and applies them to materials-related problems and opportunities.’
5G networks will help take autonomous vehicles from tentative - to widespread use.
So, which sorts of advanced materials are we talking about? These include nanomaterials that enable more efficient energy storage, lighter materials for the aerospace industry, and biodegradable nanoparticles as drug carriers within the human body.
These are just four of the 10 areas explored in the fascinating McKinsey Digital’s Tech Trends report. To read more about the rest, visit: https://www.mckinsey.com/business-functions/mckinsey-digital/our-insights/the-top-trends-in-tech
Watching plants grow in a hydroponic contraption is an education. The plants sit in foam under UV light while their roots feed on water fortified by plant feed. There is no soil. No thirst. No room for death by lazy gardener. The results, as any hydroponic enthusiast will tell you, are startling.
So, what if we were to adopt this targeted, optimised approach to our own nutrition? What would happen if he were to ditch that delicious Sunday roast in favour of a shake that contains all the vitamins and minerals your body needs? Admittedly, it sounds terrible, but people do something similar already. Many gym obsessives take protein shakes religiously to feed their bodies’ impressive musculature, while others skip meals entirely in favour of such drinks and supplements.
An organic hydroponic vegetable cultivation farm
A recent study conducted by the Cherab Foundation, which featured in the Alternative Therapies journal, concludes that nutritional supplements may also help boost our brain function. After giving 77 people a vitamin and meal replacement product called IQed Smart Nutrition, the researchers from the non-profit organisation found that the supplement boosted brain function in a range of areas and could help people with autism, apraxia, and ADHD.
Almost 84% of participants reported deficits in speech and communication prior to taking the nutritional supplements. After taking the product, more than 85% said their expressive speech had improved while 67% of respondents reported improvements in other areas including focus, language understanding, oral motor skills, and physical and behavioural health.
Overall, 64% of participants reported positive changes within two weeks. According to the Cherab Foundation, the research aims “to guide future research into the dietary interventions and potential management of neurological conditions using natural food products, vitamin and mineral supplements”.
So, what ingredients are in the supplement-infused chocolate shake that will replace the wood-fired pizza you’re due to have next Friday evening? According to IQed, its powdered chocolate offering contains everything from brown rice, apple fibres, turmeric, and green tea, to copper gluconate, amalaki, cayenne pepper, and chia seeds.
Turmeric, cayenne pepper, and chia seeds have hopped onto the superfood bandwagon in recent years.
Some will dismiss these supplements as hocus-pocus, but the potential benefits of optimised nutrition are exciting nonetheless. If some wince-inducing elixir makes us healthier, stronger and live longer, perhaps it’s worth investigating further?
The Cherub Foundation works to improve the communication skills, education, and advocacy of children on the neurological spectrum. To read more about its study, visit: https://pubmed.ncbi.nlm.nih.gov/32088673/
Farmers today are under pressure to produce more food with fewer resources and without damaging the environment around them. Faced with factors such as land pressures, soil fertility, pest management and agricultural policy, farming today is all about efficiency, time and energy saving technology, and the drive to make solutions as sustainable as possible.
This obviously poses the question: what can the agrochemical industry do to increase output on one hand and protect the environment and improve applicator safety on the other?
Formulation technology is becoming increasingly important in answering this question. By designing innovative formulations, agrochemical products can become more effective as well as safer. Without the right formulation, even the best active substance is worth nothing.
Most pesticidal active ingredients are not water soluble or water dispersible, yet the most common mode of delivery is via spray applications of aqueous dilutions. It is necessary to create a formulation of the active ingredient in a way that makes it easily dispersible in water and able to maintain stability over the application time period. Changing what goes into this formulation alongside the active ingredient is crucial in how effectively that material is delivered to where it needs to be.
Demonstration of an EC formulation.
Two of the most common types of agricultural formulations that tackle this issue are emulsifiable concentrates (ECs) and suspension concentrates (SCs). EC formulations are suited to active ingredients that are oil soluble and have low melting points. As they are purely a solubilised active ingredient in an oil or solvent with the presence of emulsifiers, they are simple to manufacture and relatively easy to stabilise. The presence of an oil also enhances the biological activity of the application, making them more efficacious in the field.
SC formulation, with an indication of what occurs upon dilution into the spray tank prior to application.
SC formulations, on the other hand, are suitable for insoluble active ingredients and those with higher melting points. Crucially, as water is the continuous phase, they are also typically safer and more convenient in use for the operator; there is an absence of dust, flammable liquids, and volatile organic compounds.
Built into each of these formulations alongside the active ingredient are formulation additives. Formulation additives, referred to as inert ingredients, are critical to provide the long-term stability to agrochemical products and their ability to mix effectively in the spray tank, making them suitable for [field spray] applications.
While the formulation type targeted is often dictated by the chemical characteristics of the active ingredient, the formulator has the ability to change every element of the spray quality characteristics and agrochemical delivery through selection of formulation additives. Changing both the formulation type and the additives within will habitually have a dramatic effect on the field efficacy of that application and subsequent yield and quality of the crop. Selecting the correct formulation additives is essential in creating a successful formulation, arguably making them as significant as the active ingredient itself.
How formulators learn to map the complex effects within formulations for improved crop protection is just one facet of today’s agriculture challenge.
Interested in learning more about how the formulation of agrochemicals plays its part in feeding the world? Visit: www.crodacropcare.com
As silicon reaches its solar ceiling, perovskite has emerged as one of the main materials of choice in the next generation of solar panels. Indeed, Oxford PV’s much anticipated perovskite-silicon solar cell could take conversion efficiency well beyond what is currently achieved on the roofs of our homes.
The benefits of perovskite are well known at this stage. It could increase the energy we harvest from the sun and improve solar cell efficiency, and its printability could make fabrication cheaper. However, as with almost everything, there are drawbacks.
According to researchers at the SPECIFIC Innovation and Knowledge Centre at Swansea University, the solvents used to control the crystallisation of the perovskite during fabrication hinder the large-scale manufacture of printed carbon perovskite cells. This is due to the toxicity and potentially psychoactive effects of these materials.
The SPECIFIC team claims to have found a way around this after discovering a non-toxic biodegradable solvent called γ-Valerolactone. They say this replacement solvent could be used without affecting solar cell performance. Furthermore, they say it is non-toxic, sustainable, and suitable for large-scale manufacturing.
Left - solvent normally used to make solar cells, which is toxic.
Right - new green solvent developed by Swansea University researchers from the SPECIFIC project
| Image Credit: Swansea University
‘This solvent problem was a major barrier, not only restricting large-scale manufacture but holding back research in countries where the solvents are banned,’ said research group leader Professor Trystan Watson. ‘We hope our discovery will enable countries that have previously been unable to participate in this research to become part of the community and accelerate the development of cleaner, greener energy.’
As the conversion efficiency of solar panels improves, cost is also key. What if you could create the same solar panels in a more cost-efficient way? That was part of the thinking behind another recent innovation in Singapore, where Maxeon Solar Technologies has created frameless, lightweight rooftop solar panels. These solar panels can be adhered directly to a roof without racking or mounting systems and allegedly perform just as well as standard solar panels.
The new Maxeon Air technology platform from Maxeon Solar Technologies
‘For close to 50 years, the solar power industry has almost exclusively used glass superstrate panel construction,’ said Jeff Waters, CEO of Maxeon Solar Technologies. ‘As solar panels have increased in size, and the cost of solar cells has been dramatically reduced, the cost of transporting, installing and mounting large glass panels has become a relatively larger portion of total system cost. With Maxeon Air technology, we can now develop products that reduce these costs while opening up completely new market opportunities such as low-load commercial rooftops.’
The idea is to use these peel-and-stick designs on low-load roofs that cannot support the weight of conventional solar systems; and they will be rolled out in 2022. Time will tell whether the innovations in Swansea and Singapore have a bearing on companies’ solar systems, but they provide more evidence of the ingenuity that is making solar power cheaper and more efficient.
We’re starting to see those silent cars everywhere. The electric vehicle evolution is gradually seeping onto our roads. Every month or two, we also seem to read about another wind power generation record in the UK, or some super solar cell. Pension funds and big corporations are coming under great pressure to divest from fossil fuels. The clean power revolution is well underway.
And yet the third biggest polluter of the planet - after power and transport - awaits the seismic shift that will shake it to its foundations. Indeed, cement production still accounts for roughly 8% of the world’s greenhouse gas emissions.
The problem is that creating cement is an energy-intense, polluting process with firing temperatures of 2,700 degrees Fahrenheit needed to create it, and plenty of CO2 released during processing.
Green cement and concrete are needed to reduce emissions in construction and other industries.
But there are signs that the processing could become cleaner. A recent report released by Market Research Future (MRFR) predicts that concrete (of which cement is a key ingredient) use could get appreciably greener over the next six years. It estimates that the global green concrete market size will grow at a 9.45% compound annual growth rate from 2020-27.
MRFR attributes this rise to several factors. First, there is a growing demand for green or recycled concrete (that incorporates waste components) within the construction industry. For builders, it enhances their environmental credentials and will increasingly become a business-savvy investment as governments seek to reduce carbon emissions.
Green building codes and the creation of energy-efficient infrastructure will also help propel this growth, and changing building regulations in massive markets including China, India, and the Middle East will result in many manufacturers looking to develop different material combinations. Increasingly, we’re seeing manufacturers turning to less energy-intensive manufacturing methods and investigating which waste materials could be used to create a greener cement or concrete that doesn’t compromise on performance.
Researchers at Chalmers University of Technology, in Sweden, have even been developing a rechargeable cement-based battery. If it ever comes to pass, this could be used to create buildings that store energy like giant batteries. Some manufacturers are also looking into the electrification of kilns, which isn’t feasible yet, and carbon capture and storage has long been mooted as a means to reduce industrial emissions.
Imagine an entire twenty storey concrete building that can store energy like a giant battery. This could be possible if Chalmers University’s cement-based rechargeable batteries come to fruition. | Image Credit: Yen Strandqvist/Chalmers University of Technology
The good news is that we don’t just have people all over the world working on low-carbon materials and manufacturing methods; experts in the UK are tackling the issue right now. On 2 June, speakers at the SCI’s free webinar, Ultra-low carbon concrete, a sustainable future, will examine some of the exciting initiatives underway.
These include an award winning, industry accepted ultra-low carbon alternative to traditional cement, which could result in CO2 savings of up to 78%, and the potential of using offsite manufacturing to provide commercial projects with a sustainable structural frame solution.
As with transport and power, cement is getting greener increment by increment. But with drastic climate change consequences dangling above us like the Sword of Damocles, now is the time for concrete action.
Register for Ultra-low carbon concrete, a sustainable future today at: https://bit.ly/33WfjkN.
Bit by bit, the green hydrogen revolution is coming to our shores. The news that a planning application has been filed for the UK’s largest electrolyser in Glasgow could be a boon for hydrogen evangelists, the local communities, and the political class.
The 20MW electrolyser will form part of the green hydrogen facility on the outskirts of Glasgow near Whitelee, the UK’s largest wind farm. The proposed project would produce up to 8 tonnes of green hydrogen each day – the equivalent of 550 return bus trips from Glasgow to Edinburgh.
If approved, the scheme would be delivered by ScottishPower, BOC, and ITM Power as part of the Green Hydrogen for Scotland Partnership. BOC would operate the facility using solar and wind power produced by Scottish Power and ITM Power would provide the all-important 20 MW electrolyser. Renewable energy would power the electrolyser, which would split the water into hydrogen and oxygen gas. The hydrogen produced by this process could then be used in various applications including transport.
Fundamentally, the people who will benefit most are the people of Glasgow, with the project aiming to provide carbon-free transport and clean air for people across the city area, while satisfying some industrial hydrogen demand. And we can all rest easy now that we know politicians will be pleased about it too, for the project coincides nicely with the United Nations 26th Climate Change Conference, which will be held in Glasgow later this year.
The new facility will be based beside a plentiful renewable energy source, Whiteless wind farm in Eaglesham Moor. | Editorial credit: Maritxu / Shutterstock.com
If all goes swimmingly, the facility will supply hydrogen for the commercial market by 2023. “Whitelee keeps breaking barriers, first the UK’s largest onshore wind farm, and soon to be home to the UK’s largest electrolyser,” says Barry Carruthers, ScottishPower’s Hydrogen Director. “The site has played a vital role in helping the UK to decarbonise and we look forward to delivering another vital form of zero carbon energy generation at the site to help Glasgow and Scotland achieve their net zero goals.”
Tumbling renewable prices
This exciting news follows on the back of some bold green hydrogen claims made in a recent Bloomberg New Energy Foundation (NEF) report: the 1H 2021 Hydrogen Levelised Cost Update. According to Martin Tengler, BloombergNEF’s Lead Hydrogen Analyst, the report authors believe the cost of renewable hydrogen could fall 85% by 2050, 17% lower than they had previously predicted. This, he says, is due to falling renewables prices.
It is becoming cheaper all the time to produce solar and wind power, which is good news for those producing green hydrogen.
Tengler also says that renewable hydrogen should be cheaper than blue hydrogen (when natural gas is split into hydrogen and CO2 via processes such as steam methane reforming) in many countries by 2030. Furthermore, Bloomberg NEF predicts that green hydrogen will be cheaper to process than natural gas in many countries by 2050.
With the prices of solar and wind power constantly tumbling, it would be no surprise to see the authors of these reports revising their projections even further in the coming years. In the mean-time, we welcome the green shoots peeking through outside Glasgow.
Many of us have contemplated buying a reconditioned phone. It might be that bit older but it has a new screen and works as well as those in the shop-front. I’m not sure, however, that any of us have thought of investing in a reconditioned liver – but it could be coming to a body near you.
Researchers based in São Paulo’s Institute of Biosciences have been developing a technique to create and repair transplantable livers. The proof-of-concept study published in Materials Science and Engineering by the Human Genome and Stem Cell Research Centre (HUG-CELL) is based on tissue bioengineering techniques known as decellularisation and recellularisation.
The organs of some donors are sometimes damaged in traffic accidents, but these may soon be transplantable if the HUG-CELL team realises its goal.
The decellularisation and recellularisation approach involves taking an organ from a deceased donor and treating it with detergents and enzymes to remove all the cells from the tissue. What remains is the organ’s extracellular matrix, containing its original structure and shape.
This extracellular matrix is then seeded with cells from the transplant patient. The theoretical advantage of this method is that the body’s immune system won’t rile against the new organ as it already contains cells from the patient’s own body, thereby boosting the chance of long-term acceptance.
However, the problem with the decellularisation process is that it removes the very molecules that tell cells to form new blood vessels. This weakens cell adhesion to the extracellular matrix. To get around this, the researchers have introduced a stage between decellularisation and recellularisation. After decellularising rat livers, the scientists injected a solution that was rich in the proteins produced by lab-grown liver cells back into the extracellular matrix. These proteins then told the liver cells to multiply and form blood vessels.
These cells then grew for five weeks in an incubator that mimicked the conditions inside the human body. According to the researchers, the results showed significantly improved recellularisation.
“It’s comparable to transplanting a ‘reconditioned’ liver, said Mayana Zatz, HUG-CELL’s principal investigator and co-author of the article. “It won't be rejected because it uses the patient’s own cells, and there’s no need to administer immunosuppressants.”
Extracellular matrix of a decellularised liver | Image Credit: HUG-CELL/USP
Obviously, there is a yawning gap between proof of concept and the operating theatre, but the goal is to scale up the process to create human-sized livers, lungs, hearts, and skin for transplant patients.
“The plan is to produce human livers in the laboratory to scale,” said lead author Luiz Carlos de Caires-Júnior to Agência FAPESP. “This will avoid having to wait a long time for a compatible donor and reduce the risk of rejection of the transplanted organ."
This technique could also be used to repair livers given by organ donors that are considered borderline or non-transplantable. “Many organs available for transplantation can’t actually be used because the donor has died in a traffic accident,” Caires-Júnior added. “The technique can be used to repair them, depending on their status.”
Even if we are at the early stages of this approach, it bodes well for future research. And for those on the organ transplant list, a reconditioned liver would be as good as a new one – complete with their very own factory settings.
Read the paper here: https://www.sciencedirect.com/science/article/abs/pii/S0928493120337814
When you live in a cold country, you think of hot days as a blessing. Air conditioning units are for those in far-away places – humid countries where the baked earth smell rises to meet you when you step off the plane.
But cooling comes at a cost. According to the UN Environment Programme, it accounts for 7% of global greenhouse gas emissions. Some of us are visual learners; so, the sheer cost of cooling really hit me when I stared up at an apartment building in Hong Kong with hundreds of air conditioning units perched above the windows like birds.
And it isn’t just the Hong Kongers feeling the heat. The cooling industry as a whole is under pressure to cut its greenhouse gas emissions. The International Energy Agency expects emissions from cooling to double by 2030 due to heat waves, population growth, urbanisation, and the growing middle class. By 2050, it forecasts that space cooling will consume as much electricity as China and India do today.
Air conditioning units cling to a building
All of this was captured by the Cooling Suppliers: Who's Winning the Race to Net Zero report released by the Race to Zero campaign, the Kigali Cooling Efficiency Program (K-CEP), Carbon Trust and other partners in the UN Environment Programme-hosted Cool Coalition.
This report's authors found that only five of the 54 cooling companies they assessed have committed to net-zero targets. The document outlines three areas that must be addressed on the Cooling Climate Pathway: super-efficient appliances, ultra-low global warming refrigerants, and the widespread adoption of passive cooling measures such as clever home design and urban planning.
So, while builders adjust window sizes, introduce trees for shading, and choose materials (such as terracotta cooling systems) thoughtfully to temper the sun’s gaze, others are availing of different methods.
For example, the COP26 (the 2021 UN Climate Change Conference) Champions Team has just released its Net Zero Cooling Action Plan that includes a Cool Calculator tool to help companies and governments run simple calculations to see where they could decarbonise their cooling systems. Similarly, the UK's Environmental Investigation Agency (EIA) has launched a net-zero cooling product guide that showcases energy-efficient products run on natural refrigerants.
Green walls are one of many passive cooling approaches used to reduce our reliance on mechanical systems.
However, it’s clear that the softly-softly approach won’t suffice. The EIA has called on governments to do more to encourage organisations to adopt sustainable cooling, to make concrete policy commitments, and speed-up the phase-out of climate-warming refrigerants such as hydrofluorocarbons.
“The development and expansion of net-zero cooling is a critical part of our Race to Zero emissions,” said Nigel Topping, UK High Level Champion for COP26. “In addition to technological breakthroughs and ambitious legislation, we also need sustainable consumer purchasing to help deliver wholesale systems change.”
We all love the technological panacea – innovations that will cure all the climate ills we have inflicted on the world. But the solution will also involve stodgy government regulations and changing consumer habits, and a reliance on the continued fall in renewable power generation.
For those in traditionally cooler climes, it’s no longer someone else’s problem. It was a balmy 22°C in London this week and we’re not even in April yet. So, it’s certainly time to turn up the heat on the cooling industry.
Every day, there are subtle signs that machine learning is making our lives easier. It could be as simple as a Netflix series recommendation or your phone camera automatically adjusting to the light – or it could be something even more profound. In the case of two recent machine-learning developments, these advances could make a tangible difference to both microscopy, cancer treatment, and our health.
The first is an artificial intelligence (AI) tool that improves the information gleaned from microscopic images. Researchers at the University of Gothenburg have used this deep machine learning to enhance the accuracy and speed of analysis.
The tool uses deep learning to extract as much information as possible from data-packed images. The neural networks retrieve exactly what a scientist wants by looking through a huge trove of images (known as training data). These networks can process tens of thousands of images an hour whereas some manual methods deliver about a hundred a month.
Machine learning can be used to follow infections in a cell.
In practice, this algorithm makes it easier for researchers to count and classify cells and focus on specific material characteristics. For example, it can be used by companies to reduce emissions by showing workers in real time whether unwanted particles have been filtered out.
“This makes it possible to quickly extract more details from microscope images without needing to create a complicated analysis with traditional methods,” says Benjamin Midtvedt, a doctoral student in physics and the main author of the study. “In addition, the results are reproducible, and customised. Specific information can be retrieved for a specific purpose."
The University of Gothenburg tool could also be used in health care applications. The researchers believe it could be used to follow infections in a cell and map cellular defense mechanisms to aid the development of new medicines and treatments.
Machine learning by colour
On a similar thread, machine learning has been used to detect cancer by researchers from the National University of Singapore. The researchers have used a special dye to colour cells by pH and a machine learning algorithm to detect the changes in colour caused by cancer.
The researchers explain in their APL Bioengineering study that the pH (acidity level) of a cancerous cell is not the same as that of a healthy cell. So, you can tell if a cell is cancerous if you know its pH.
With this in mind, the researchers have treated cells with a pH-sensitive dye called bromothymol blue that changes colour depending on how acidic the solution is. Once dyed, each cell exudes its unique red, green, and blue fingerprint.
By isolating a cell’s pH, researchers can detect the presence of cancer.
The authors have also trained a machine learning algorithm to map combinations of colours to assess the state of cells and detect any worrying shifts. Once a sample of the cells is taken, medical professionals can use this non-invasive method to get a clearer picture of what is going on inside the body. And all they need to do all of this is an inverted microscope and a colour camera.
“Our method allowed us to classify single cells of various human tissues, both normal and cancerous, by focusing solely on the inherent acidity levels that each cell type tends to exhibit, and using simple and inexpensive equipment,” said Chwee Teck Lim, one of the study’s authors.
“One potential application of this technique would be in liquid biopsy, where tumour cells that escaped from the primary tumour can be isolated in a minimally invasive fashion from bodily fluids.”
The encouraging sign for all of us is that these two technologies are but two dots on a broad canvas, and machine learning will enhance analysis. There are certainly troubling elements to machine learning but anything that helps hinder disease is to be welcomed.
Machine Learning-Based Approach to pH Imaging and Classification of Single Cancer Cells:
https://aip.scitation.org/doi/10.1063/5.0031615
Quantitative Digital Microscopy with Deep Learning:
https://aip.scitation.org/doi/10.1063/5.0034891
What do grape stalks, pineapple leaves, corn cobs, rice husks, sheep’s wool, and straw have in common? Apart from being natural materials, they have all been used to insulate homes. Increasingly, people are turning towards natural, sustainable materials as climate change and waste have become bigger problems.
Existing building insulation materials such as synthetic rock wool are excellent at keeping our homes warm in winter, but the conversation has moved beyond thermal performance. Energy use, re-usability, toxicity, and material disposal are all live considerations now, especially with regulations and emissions targets tightening. So, rock wool might perform better than straw bale insulation but straw is biodegradable, reusable, easy to disassemble, and doesn’t require large amounts of energy to process.
Sheep’s wool and hemp insulation have also become attractive to homeowners and housebuilders alike, but an even more encouraging prospect is the use of waste materials to create next generation insulation. In this spirit, researchers at Flinders University in Adelaide, Australia, have taken waste cooking oil, wool offcuts, and sulphur to process a novel housing insulation material.
Recycled paper is one of many waste materials that has found its way into domestic insulation.
To make this composite, they followed several stages. In the first stage of the synthesis, the researchers used inverse vulcanisation to create a polysulphide polymer from canola oil triglyceride and sulphur. They then mixed this powdered polymer with wool and coated the fibres through electrostatic attraction. This mixture was compressed through mild heating to provoke S−S metathesis in the polymer and bind the wool. The wool bolsters the tensile strength of the material, makes it less flammable, and provides excellent insulation. The result is a sustainable building material that fulfils its function without damaging the environment.
For Associate Professor Justin Chalker, the lead author of this study, this work provides an ideal jumping-off point. “The promising mechanical and insulation properties of this composite bodes well for further exploration in energy saving insulation in our built environment,” he said.
Sustainable transformation
It is clear that ventures like the one in Adelaide will continue to sprout all over the world. After all, necessity dictates that we change the way we build our homes and treat materials.
A recent report from Emergen Research predicts that the global insulation materials market will be worth US $82.96 billion (£59.78 billion) by 2027. The same report was also at pains to mention that the increasing demand for reduced energy consumption in buildings will be a significant factor in influencing industry growth.
“Market revenue growth is high and expected to incline rapidly going ahead due to rising demand for insulation materials... to reduce energy consumption in buildings,” it said. One of the main reasons given for this increased green building demand was stricter environmental regulations.
And Emergen isn’t the only organisation feeling the ground moving. Online roofing merchant Roofing Megastore, which sells more than 30,000 roofing materials, has detected a shift towards environmentally friendly materials, with many homeowners sourcing these products themselves.
Rock wool insulation panels have come under greater scrutiny in recent times.
Having analysed two years of Google search data on sustainable building materials, the company found that synthetic roof tiles are generating the most interest from the public. Like the Flinders insulation, these roof tiles make use of waste materials, in this case recycled limestone and plastic. And you don’t need to look far down the list to find sustainable insulation materials, with sheep’s wool insulation in 9th place, wood fibre insulation in 10th, and hemp insulation in 12th.
Over time, the logic of the progression towards natural, less energy-intensive building materials will become harder to ignore. “Traditional materials such as synthetic glass mineral wool offer high levels of performance but require large amounts of energy to produce and must be handled with care while wearing PPE,” the company noted. “Natural materials such as hemp or sheep’s wool, however, require very little energy to create and can be installed easily without equipment.”
So, the next time you look down at your nutshells, spent cooking oil, or tattered woollen sweater, think of their potential. In a few years, these materials could be sandwiched between your walls, keeping you warm all winter.
Insulating composites made from sulphur, canola oil, and wool (2021): https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cssc.202100187?af=R
A completely clean, renewable energy system that can be produced locally and that can easily power heat, energy storage and transportation, and travel — that's the future that promoters of a hydrogen economy envisage.
If it sounds a bit like rocket science, that's because it is. Hydrogen is what's used to fuel rockets — that’s how powerful it is. In fact, it’s three times more powerful as a fuel than gas or other fossil-based sources. And, after use, it’s frequently converted to drinking water for astronauts.
US President Joe Biden has highlighted the potential of hydrogen in his ambitious plans for economic and climate recovery and a number of recent reports have been encouraging about hydrogen’s breakthrough moment, including McKinsey and Company (Road Map to a US Hydrogen Economy, 2020) and the International Energy Agency.
Hydrogen fuel cells provide a tantalising glimpse into our low-carbon future
The McKinsey report claims that, by 2030, the hydrogen sector could generate 700,000 jobs and $140bn in revenue, growing to 3.4 million jobs and $750bn by 2050. It also believes it could account for a 16% reduction in CO2 emissions, a 36% reduction in NOx emissions, and supply 14% of US energy demand.
So how does it work?
Simply put, hydrogen fuel cells combine hydrogen and oxygen atoms to produce electricity. The hydrogen reacts with oxygen across an electrochemical cell and produces electricity, water, and heat.
This is what gets supporters so excited. In theory, hydrogen is a limitless, incredibly powerful fuel source with no direct emissions of pollutants or greenhouse gases.
So what's the problem?
Right now, there are actually a few problems. The process relies on electrolysis and steam reforming, which are extremely expensive. The IEA estimates that to produce all of today’s dedicated hydrogen output from electricity would require 3,600TWh, more than the total annual electricity generation of the European Union.
Moreover, almost 95% of hydrogen currently is produced using fossil fuels such as methane, natural gas, or coal (this is called "grey hydrogen"). Its production is responsible for annual CO2 emissions equivalent to those of Indonesia and the United Kingdom combined. In addition, its low density makes it difficult to store and transport — it must be under high pressure at all times. It’s also well-known for being highly flammable — its use as a fuel has come a long way since the Hindenburg Disaster but the association still makes many people nervous.
A Hydrogen refuelling station Hafencity in Hamburg, Germany. Infrastructure issues must be addressed if we are to see more hydrogen-fuelled vehicles on our roads. | Image credit: fritschk / Shutterstock.com
So there are quite a few problems. What’s the good news?
In the last few years, we've seen how rapidly investment, innovation, and infrastructure policy can completely transform individual renewable energy industries. For example, the IEA analysis believes the declining costs of renewables and the scaling up of hydrogen production could reduce the cost of producing hydrogen from renewable electricity 30% by 2030.
Some of the issues around expense could be resolved by mass manufacture of fuel cells, refuelling equipment, and electrolysers (which produce hydrogen from electricity and water), made more likely by the increased interest and urgency. Those same driving forces could improve infrastructural issues such as refuelling stations for private and commercial vehicles, although this is likely to require coordination between various stakeholders, including national and local governments, industry, and investors.
The significant gains in renewable energy mean that “green” hydrogen, where renewable electricity powers the electrolysis process, is within sight.
The IEA report makes clear that international co-operation is “vital” to progress quickly and successfully with hydrogen energy. R&D requires support, as do first movers in mitigating risks. Standards need to be harmonised, good practice shared, and existing international infrastructure built on (especially existing gas infrastructure).
If hydrogen can be as efficient and powerful a contributor to a green global energy mix as its proponents believe, then it's better to invest sooner rather than later. If that investment can help power a post-Covid economic recovery, even better.
The Organisation for Economic Cooperation and Development (OECD) defines the Blue Economy as ‘all economic sectors that have a direct or indirect link to the oceans, such as marine energy, coastal tourism and marine biotechnology.’ Other organisations have their own definitions, but they all stress the economic and environmental importance of seas and oceans.
Header image: Our oceans are of economic and environmental importance
To this end there are a growing number of initiatives focused on not only protecting the world’s seas but promoting economic growth. At the start of 2021 the Asian Development Bank (ADB) and the European Investment Bank (EIB) joined forces to support clean and sustainable ocean initiatives in the Asia-Pacific region, and ultimately contribute to achieving Sustainable Development Goals and the climate goals of the Paris Agreement.
Both institutions will finance activities aimed at promoting cleaner oceans ‘through the reduction of land-based plastics and other pollutants discharged into the ocean,’ as well as projects which improve the sustainability of all socioeconomic activities that take place in oceans, or that use ocean-based resources.
ADB Vice-President for Knowledge Management and Sustainable Development, Bambang Susantono, said ‘Healthy oceans are critical to life across Asia and the Pacific, providing food security and climate resilience for hundreds of millions of people. This Memorandum of Understanding between the ADB and EIB will launch a framework for cooperation on clean and sustainable oceans, helping us expand our pipeline of ocean projects in the region and widen their impacts’.
The blue economy is linked to green recovery
In the European Union the blue economy is strongly linked to the bloc’s green recovery initiatives. The EU Blue Economy Report, released during June 2020, indicated that the ‘EU blue economy is in good health.’ With five million people working in the blue economy sector during 2018, an increase of 11.6% on the previous year, ‘the blue economy as a whole presents a huge potential in terms of its contribution to a green recovery,’ the EU noted. As the report was launched, Mariya Gabriel, Commissioner for Innovation, Research, Culture, Education and Youth, responsible for the Joint Research Committee said; ‘We will make sure that research, innovation and education contribute to the transition towards a European Blue Economy.’
The impact of plastics in oceans is well known and many global initiatives are actively tackling the problem. At the end of 2020 the World Economic Forum and Vietnam announced a partnership to tackle plastic pollution and marine plastic debris. The initiative aims to help Vietnam ‘dramatically reduce its flow of plastic waste into the ocean and eliminate single-use plastics from coastal tourist destinations and protected areas.’ Meanwhile young people from across Africa were congratulated for taking leadership roles in their communities as part of the Tide Turners Plastic Challenge. Participants in the challenge have raised awareness of the impact of plastic pollution in general.
But it isn’t just the health of our oceans that governments and scientists are looking at. There is growing interest in the minerals and ore that could potentially be extracted via sea-bed mining. The European Commission says that the quantity of minerals occupying the ocean floor is potentially large, and while the sector is small, the activity has been identified as having the potential to generate sustainable growth and jobs for future generations. But adding a note of caution, the Commission says, ‘Our lack of knowledge of the deep-sea environment necessitates a careful approach.’ Work aimed at shedding light on the benefits, drawbacks and knowledge gaps associated with this type of mining is being undertaken.
With the push for cleaner energy and the use of batteries, demand for cobalt will rise, and the sea-bed looks to have a ready supply of the element. But, the World Economic Forum points out that the ethical dimensions of deep-sea cobalt have the potential to become contentious and pose legal and reputational risks for mining companies and those using cobalt sourced from the sea-bed.
Energy will continue to be harnessed from the sea.
But apart from its minerals, the ocean’s ability to supply energy will continue to be harnessed through avenues such as tidal and wind energy. During the final quarter of 2020, the UK Hydrographic Office launched an Admiralty Marine Innovation Programme. Led by the UK Hydrographic Office, the programme gives innovators and start-ups a chance to develop new solutions that solve some of the world’s most pressing challenges as related to our oceans.
The UK’s Blue Economy is estimated to be worth £3.2 trillion by the year 2030. Marine geospatial data will be important in supporting this growth by enabling the identification of new areas for tidal and wind energy generation, supporting safe navigation for larger autonomous ships, which will play a vital role in mitigating climate change, and more.
Where once a country might have wanted to strike gold, now hitting upon a hydrocarbon find feels like a prize. But finding a hydrocarbon is only the beginning of the process and might not be worth it — as Lebanon is discovering.
First, a little background: for some time, Lebanon has been experiencing an energy crisis. Without resources of their own, the industry (which is government-owned) is reliant on foreign imports, which are expensive. Electricity in early 2020 was responsible for almost 50% of Lebanon's national debt. Major blackouts were common.
This contributed to a spiralling financial crisis, prompting public protests and riots as the middle class disappeared and even wealthier citizens struggled. Before Covid-19 and the devastating August 2020 blast in Beirut, Lebanon was in crisis.
The idea that the country might be able to switch from foreign oil to local gas was understandably appealing, especially when a major find was literally right there on the Lebanese shore. In 2019, a consortium of Israeli and US firms discovered more than 8tcm of natural gas in several offshore fields in the Eastern Mediterranean, much of it in Lebanese waters.
A hydrocarbon find off the Beirut coast has failed to live up to its early promise.
But a find is only the beginning. With trust in Lebanese politicians low (the country ranks highly in most government corruption indexes) and a system that has repeatedly struggled to deliver a stable government, there are additional difficulties, not least a delay in the licensing rounds and a lack of trust — both internally, from citizens, and externally, from potential bidders. Meanwhile, Lebanon's neighbours race ahead to exploit their own finds, which ratchets up tensions.
Amid all that, a drilling exploration managed to go ahead last summer. But the joint venture between Total, ENI, and Novatek, which operated a well 30km offshore Beirut and drilled to approximately 1,500 metres, did not bring back the hoped-for results. The results confirmed the presence of a hydrocarbon system generally but did not encounter any reservoirs of the Tamar formation, which was the target.
Offshore exploration is a long process, with a lot of challenges and uncertainties and Ricardo Darré, Managing Director of Total E&P Liban, said afterwards, "Despite the negative result, this well has provided valuable data and learnings that will be integrated into our evaluation of the area". But the faith national politicians have long put in the hydrocarbon find, selling it as an answer to all Lebanon's problems, seems to have only worsened the domestic situation since.
And domestic politics is just the start of the problems…
Unlike other countries in the Middle East, Lebanon has no pipeline infrastructure of its own.
Israel, Egypt, and Jordan already have pipelines, which go to Italy. Turkey is working with Libya on a pipeline. Lebanon has no pipeline infrastructure of its own yet, although Russia has storage facilities and pipelines in the country and an eye on possible competition in the gas market.
None of that is an issue if the supply is intended for domestic use but that might not be profitable enough for investors and the Lebanese government would struggle to underwrite production on its own. Cyprus has encountered similar issues exploiting its share of the find.
Lebanon has also set an ambitious goal of having 30% of domestic energy mix sourced from renewable energy by 2030. The hoped-for gas was intended to support the renewable energy mix but, with the clock ticking, it might be that priorities shift to focusing on renewables. The Covid-19 pandemic will significantly impact the budgets of drilling companies and the push for renewable energy, both from governments and investors, seems to be growing as a way to boost economic recovery.
It may be that, after all the excitement around the hydrocarbon find, Lebanon starts to look elsewhere for its energy provision.
The world’s biggest ever survey of public opinion on climate change was published on 27th January, covering 50 countries with over half of the world’s population, by the United Nations Development Programme (UNDP) and the University of Oxford. Of the respondents, 64% believe climate change is a global emergency, despite the ongoing Covid-19 pandemic, and sought broader action to combat it. Earlier in the month, US President Joe Biden reaffirmed the country's commitment to the Paris Agreement on Climate Change.
It is possible that the momentum, combined with the difficulties many countries currently face, may make many look again to geoengineering as an approach. Is it likely that large scale engineering techniques could mitigate the damage of carbon emissions? And is it safe to do so or could we be exacerbating the problem?
The term has long been controversial, as have many of the suggested techniques. But it would seem that some approaches are gaining more mainstream interest, particularly Carbon Dioxide Removal (CDR) and Solar Radiation Modification (SRM), which the 2018 Intergovernmental Panel on Climate Change (IPCC) report for the UN suggested were worth further investigation (significantly, it did not use the term "geoengineering" and distinguished these two methods from others).
One of the most covered CDR techniques is Carbon Capture and Storage (CCS) or Carbon Capture, Utilisation, and Storage (CCUS), the process of capturing waste carbon dioxide, usually from carbon intensive industries, and storing (or first re-using) it so it will not enter the atmosphere. Since 2017, after a period of declining investment, more than 30 new integrated CCUS facilities have been announced. However, there is concern among many that it will encourage further carbon emissions when the goal should be to reduce and use CCS to buy time to do so.
CDR techniques that utilise existing natural processes of natural repair, such as reforestation, agricultural practices that absorb carbon in soils, and ocean fertilisation are areas that many feel could and should be pursued on a large scale and would come with ecological and biodiversity benefits, as well as fostering a different, more beneficial relationship with local environments.
A controversial iron compound deposition approach has been trialled to boost salmon numbers and biodiversity in the Pacific Ocean.
The ocean is a mostly untapped area with huge potential and iron fertilisation is one very promising area. The controversial Haida Salmon Corporation trial in 2012 is perhaps the most well-known example and brings together a lot of the pros and cons frequently discussed in geoengineering — in many ways, we can see it as a microcosm of the bigger issue.
The trial deposited 120 tonnes of iron compound in the migration routes of pink and sockeye salmon in the Pacific Ocean 300k west of Haida Gwaii over a period of 30 days, which resulted in a 35,000km2, several month long phytoplankton bloom that was confirmed by NASA satellite imagery. That phytoplankton bloom fed the local salmon population, revitalising it — the following year, the number of salmon caught in the northeast Pacific went from 50 million to 226 million. The local economy benefited, as did the biodiversity of the area, and the increased iron in the sea captured carbon (as did the biomass of fish, for their lifetimes).
Small but mighty, phytoplankton are the laborers of the ocean. They serve as the base of the food web.
But Environment Canada believes the corporation violated national environmental laws by depositing iron without a permit. Much of the fear around geoengineering is how much might be possible by rogue states or even rogue individuals, taking large scale action with global consequences without global consent.
The conversation around SRM has many similarities — who decides that the pros are worth the cons, when the people most likely to suffer the negative effects, with or without action, are already the most vulnerable? This is a concern of some of the leading experts in the field. Professor David Keith, an expert in the field, has publicly spoken about his concern around climate change and inequality, adding after the latest study that, "the poorest people tend to suffer most from climate change because they’re the most vulnerable. Reducing extreme weather benefits the most vulnerable the most. The only reason I’m interested in this is because of that."
But he doesn't believe anywhere near sufficient research has been done into the viability of the approach or the possible consequences and cautions that there is a need for "an adequate governance system in place".
There is no doubt that the research in this field is exciting but there are serious ethical and governance problems to be dealt with before it can be considered a serious component of an emissions reduction strategy.
We are increasingly conscious of the need to recycle waste products, but it is never quite so easy as rinsing and sorting your waste into the appropriate bins, especially when it comes to plastic.
Despite our best intentions, only around 16% of plastic is recycled into new products — and, worse, plastics tend to be recycled into low quality materials because transformation into high-value chemicals requires substantial amounts of energy, meaning the choices are either downcycling or prohibitively difficult. The majority of single-use plastics end up in landfills or abandoned in the environment.
This is a particular problem when it comes to polyolefins such as polyethylene (PE) and polypropylene (PP), which use cheap and readily available raw materials. Approximately 380 million tonnes of plastics are generated annually around the world and it is estimated that, by 2050, that figure will be 1.1 billion tonnes. Currently, 57% of this total are polyolefins.
Why are polyolefins an issue? The strong sp3 carbon–carbon bonds (essentially long, straight chains of carbon and hydrogen atoms) that make them useful as a material also make them particularly difficult to degrade and reuse without intensive, high energy procedures or strong chemicals. More than most plastics, downcycling or landfill disposal tend to be the main end-of-life options for polyolefins.
Polyethylene is used to make plastic bags and packaging.
Now, however, a team of scientists from MIT, led by Yuriy Román-Leshkov, believe they may have made a significant step towards solving this problem.
Previous research has demonstrated that noble metals, such as zirconium, platinum, and ruthenium can help split apart short, simple hydrocarbon chains as well as more complicated, but plant-based lignin molecules, in processes with much lower temperatures and energy.
So the team looked at using the same approach for the long hydrocarbon chains in polyolefins, aiming to disintegrate the plastics into usable chemicals and natural gas. It worked.
First, they used ruthenium-carbon nanoparticles to convert more than 90% of the hydrocarbons into shorter compounds at 200 Celsius (previously, temperatures of 430–760 Celsius were required).
Next, they tested their new method on commercially available, more complex polyolefins without pre-treatment (an energy intensive requirement). Not only were the samples completely broken down into gaseous and liquid products, the end product could be selected by tuning the reaction, yielding either natural gas or a combination of natural gas and liquid alkanes (both highly desirable) as preferred.
Polypropylene is used in bottle caps, houseware, and other packaging and consumer products.
The researchers believe that an industrial scale use of their method could eventually help reduce the volume of post-consumer waste in landfills by recycling plastics to desirable, highly valuable alkanes — but, of course, it's not that simple. The team says that more research into the effects of moisture and contaminants in the process is required, as well as product removal strategies to decrease the formation of light alkanes which will be critical for the industrialisation of this reaction.
However, they believe the path they're on could lead to affordable upcycling technology that would better integrate polyolefins into the global economy and incentivise the removal of waste plastics from landfill and the environment.
More about the study can be read here:
https://pubs.acs.org/doi/full/10.1021/jacsau.0c00041
The Organisation for Economic Cooperation and Development (OECD) has published its Science Technology and Innovation Outlook 2021: Time of Crises and Opportunity report.
Published at the beginning of 2021, the report focuses on the ‘unparalleled mobilisation of the scientific and innovation community’ in response to the covid-19 pandemic. The report indicates that newly funded research initiatives have been established by public research agencies and organisations, private foundations and charities, while the health sector has similarly invested in an array of research programmes worth billions of dollars in record time.
The pandemic has led an unprecedented mobilisation of the scientific and innovation community
However, the report also exposes gaps in overall system resilience to future crises. ‘It’s a wake-up call that highlights the need to recalibrate science, technology and innovation (STI) policies, so that they better orient research and innovation efforts towards sustainability, inclusivity and resiliency goals,’ the report asserts.
Highlighting the rapid response by governments around the world, the report indicates that in the first few months of the pandemic, national research funding bodies spent around $5 billion on emergency financial support. This includes $300 million in Asia-Pacific, excluding China, over $850 million in Europe and more than $3.5 billion in North America. At the same time, research efforts led to around 75,000 scientific publications on covid-19 being released between January and November 2020, the report says. The largest share came from the US, followed by China and the UK. Research databases and scientific publishers removed paywalls so that covid-19 related information could be quickly shared.
Research efforts led to around 75,000 scientific publications on covid-19 being released between January and November 2020
‘These developments mark important changes that could accelerate the transition to a more open science in the longer run,’ the report says. It is also noted that not only have researchers continued their work with more than three quarters of scientists indicating that they had shifted to working from home at some point in 2020, but almost two thirds experienced, or expected to see, an increase in the use of digital tools for research as a consequence of the crisis. The report also mentions the contribution of the general public, with so called ‘frugal innovations’ in response to shortages of medical equipment and emergency supplies.
Looking to the future of the research community, the report says that postgraduate training regimes require reform to support a diversity of career paths. ‘The crisis has shown that the need for STI expertise is not limited to the public laboratory; it is also important for business, government and NGOs […] Reforming PhD and post-doctoral training to support a diversity of career paths is essential for improving societies’ ability to react to crises like covid-19 and to deal with long-term challenges like climate change that demand science-based responses […] There has been a 25% increase in the number of people with PhDs in OECD countries over the past decade with no corresponding increase in academic posts. The pandemic is expected to make matters worse, more than half of the scientists participating in the OECD Science Flash Survey expect the crisis to negatively affect their job security and career opportunities,’ the report says.
Post-graduate training regimes require reform to support a diversity of career paths
While still in the midst of the pandemic, the report stresses that STI policies now need to be reoriented to tackle the challenges of sustainability, inclusivity and resiliency. ‘In the short-term governments should continue their support for science and innovation activities that aim to develop solutions to the pandemic and mitigate its negative impacts, while paying attention to its uneven distributional effects. Science for policy will remain in the spotlight as governments seek to strike the right balance in their response to covid-19. This will effect public perceptions of science that could have long term implications for science-society relations.’
The report concludes that governments now have the task of developing public sector capabilities to deliver more ambitious STI policy. This will require engagement from stakeholders and citizens in order to capture a diversity of knowledge and values.
DOI:10.1787/75f79015-en
Galen (129-216 CE) is one of the most famous and influential medical practitioners in history but he was also a scientist, an author, a philosopher, and a celebrity. He wrote hundreds of treatises, travelled and studied widely, was the physician to three emperors, and left a legacy of scientific thought that lasted for fifteen hundred years — even today, his work has an influence.
Header image Editorial credit: Eray Adiguzel / Shutterstock.com
He grew up in Pergamum, an intellectual centre of the Mediterranean world, in a wealthy family that encouraged him to pursue academia and funded his travels to learn in the best environments available, acquiring the latest techniques in medicine and healing.
He understood that diet, exercise, and hygiene were essential for good health and put that into practice in the four years he spent working for the High Priest of Pergamum's Gladiator School. This was a high profile and high pressure role and we know he reduced the death rate dramatically in his four years there. The recommendation he got helped secure him a position in Rome, capital of the empire.
He was not popular in the city — at one point, he seems to have been chased out by the local physicians, who strenuously disagreed with his methods — but he was eventually summoned by the emperor Marcus Aurelius to be his personal physician. He was described by the emperor as, “First among doctors and unique among philosophers".
Galen; Line engraving | Credit: Wellcome Images, Wikimedia Commons
Galen continued to navigate the difficult political environment of the imperial capital and was personal physician to two more emperors, while publishing prolifically and becoming one of the most well-known figures in the Roman Empire. Much of his work is lost to us but we still know a great deal about him, including that he had a flair for showmanship and controversy.
In the Greek world where he grew up, dissections had been common — of animals and humans. In Rome, this was not the case. In fact, human dissections were banned across the empire shortly before Galen arrived in the city. Undaunted, he gave a number of public anatomical demonstrations using pigs, monkeys, sheep, and goats to show his new city what they were missing (this was one of many incidents that contributed to local dislike of his methods as well as his increasing fame).
His legacy was huge, both because he recorded and critiqued the work of others in his field and because of the huge volumes of his own observations and theories. His texts were the foundation for much of medical education in the Islamic, Byzantine, and European worlds until the 17th Century.
The ban on human dissection likely limited his progress in some areas and many of his theories have (eventually) been disproved, such as the theory of the four humours — blood, black bile, yellow bile, and phlegm — based on Hippocrates' system and elaborated, as well as the efficacy of bloodletting.
Galen observed that cataracts could be removed.
In other areas, however, he was remarkably successful. He observed that the heart has four valves that allow blood to flow in only one direction, that a patient's pulse or urine held clues to their disease, that urine forms in kidneys (previously thought to be the bladder), that arteries carry liquid blood (previously thought to be air), that cataracts could be removed from patients' eyes, among others. He also identified seven of the 12 cranial nerves, including the optic and acoustic nerves.
His focus on practical methods such as direct observation, dissection, and vivisection is obviously still relevant to modern medical research. Indeed, scientists who disproved his theories, such as Andreas Vesalius and Michael Servetus in the 16th century, did so using Galen's own methods.
The study of his work remains hugely important to the history and understanding of medicine and science, as well as the ancient world. The Galenic formulation, which deals with the principles of preparing and compounding medicines in order to optimise their absorption, is named after him.
A year after the world was put on alert about the rapidly spreading covid-19 virus, mass vaccination programmes are providing a welcome light at the end of the tunnel.
However, for many people vaccination remains a concern. A World Economic Forum – Ipsos survey: Global Attitudes on a Covid-19 Vaccine, indicates that while an increasing number of people in the US and UK plan to get vaccinated, the intent has dropped in South Africa, France, Japan and South Korea. The survey was conducted in December 2020, following the first vaccinations in the US and the UK.
This study shows that overall vaccination intent is below 50% in France and Russia. ‘Strong intent’ is below 15% in Japan, France and Russia.
Many cities are still in lockdown
Between 57% and 80% of those surveyed cited concerns over side effects as a reason for not getting a covid-19 vaccination. Doubts over the effectiveness of a vaccine was the second most common reason cited in many countries, while opposition to vaccines in general was mentioned by around 25% those who will refuse a vaccination.
The survey was conducted among 13,542 adults aged 18–74 in Canada, South Africa, and the US, while those surveyed in Australia, Brazil, China, France, Germany, Italy, Japan, Mexico, Russia, South Korea, Spain and the UK were aged 16–74.
A previous survey, Global Attitudes on a Covid-19 Vaccine carried out in July and August 2020, indicated that 74% of those surveyed intended to get vaccinated. At that time the World Economic Forum said that this majority could still fall short of the number required to ‘beat covid-19.’
Commenting on the newest data Arnaud Bernaert, Head of Health and Healthcare at the World Economic Forum said; ‘As vaccinations roll out, it is encouraging to see confidence improve most in countries where vaccines are already made available. It is critical that governments and the private sector come together to build confidence and ensure that manufacturing capacity meets the global demand.’
World Economic Forum-Ipsos Survey indicates a rise in number of people in US and UK intending to get vaccinated.
With the imperative now to move towards some sort of ‘normality’, as well as getting economies moving, fears over vaccination need to be allayed. However, what also needs to be considered is what underlies those fears. Misinformation, no doubt, has a part to play. This highlights a lack of trust in governments and a sector that has worked tirelessly to develop vaccines in record time.
As different companies bring their vaccines to the market, care now needs to be taken to reassure people around the world that whichever manufacturer’s vaccine they are given, they are in safe hands. As any adverse reactions occur – an inevitability with any vaccine rollout – these ought to be made known to the wider public by companies and governments as soon as it is feasible, preventing space for the spread of rumour and misinformation, which could undo the hard work of the scientists, businesses and governments bringing vaccines to the public.

Researchers have worked tirelessly to bring vaccines to the market
Waking up after a night of overindulgence on food and wine and realising you don’t have a headache is very satisfying. But realising, soon afterwards, you have heartburn can bring your mood down rapidly.
After years of discussion and argument around Brexit, the UK woke up to find that a Trade and Cooperation Agreement between the UK and the EU been reached. A major headache had been avoided.

UK Businesses have a new trading landscape
However, the UK chemicals sector soon realised that after pulling back the curtains and taking a look at the new trading landscape, a feeling of heartburn was rising. The chemical sector’s regulatory obligation now requires that it establishes a UK-REACH system. The deal negotiated means that the UK has no access to the data it submitted to the EU’s REACH database.
In effect, the UK chemical sector has to populate the UK-REACH system from scratch. This will require an array of steps possibly including testing and renegotiating data sharing with other companies. According to the Chief Executive of the Chemical Industries Association (CIA), Steve Elliot, this is set to burn a £1 billion hole in the UK chemical sector’s pocket.
‘Failure to secure access to what has been a decade’s worth of investment by UK chemical businesses in data for EU REACH will leave the industry facing a bill of more than £1 billion in unnecessarily duplicating that work for a new UK regime,’ said Elliot in a statement on 24 December 2020, the day that the UK government excitedly announced the new trade deal.

UK-REACH could cost more than £1 billion
As a slightly belated Christmas gift, and perhaps just taking the edge off the heartburn, the UK government’s Environment Minister, Rebecca Pow announced, on 31 December, that the UK-REACH IT system was up and running. Pow said that the government had worked closely with partners, industry and stakeholders developing the IT system to manage the UK’s chemicals industry.
‘Having our own independent chemicals regulatory framework will ensure that we make decisions that best reflect the UK’s needs while maintaining some of the highest chemical standards in the world,’ she said.
But will these high standards do what REACH was set up for in the first place, and protect human health and the environment? According to CHEM Trust, a UK-German charity focused on preventing man-made chemicals from causing long term damage to wildlife or humans, the deal does not go far enough.
Critiquing the outcome, Michael Warhurst, Executive Director of CHEM Trust said, ‘CHEM Trust’s initial assessment is that this agreement does not adequately protect human health and the environment in the UK from hazardous chemicals. This is because it doesn’t retain UK access to the EU’s chemicals regulation system REACH. The agreement includes an annex on chemicals, but does not facilitate the type of close cooperation with the EU post-Brexit that civil society groups such as CHEM Trust, and also the chemicals and other industries are seeking.’
But on a positive note, Warhurst added; ‘The deal […] commits the UK to not regress from current levels of protection, includes a rebalancing procedure which could increase protection on both sides and offers a platform on which a closer partnership could be negotiated in the future.’
No one doubts that there is still much to be digested, along with those left over Christmas chocolates that nobody really likes, regarding the UK-EU Free Trade Agreement. ‘Although this Free Trade Agreement represents a mixed bag for our industry,’ said the CIA’s Elliot, ‘we shouldn’t underestimate the huge value that a deal brings in terms of certainty.’

2021: A year to look forward to
As people return to their desks after the Christmas break, one might dare to hope that the heartburn can be quelled with a dose of optimism after the challenging year that has just passed. With this as a basis, along with eventually emerging from the global pandemic, Elliot believes 2021 should be ‘a year to look forward to’.